Venus resembles the Earth in many ways; it is roughly the same size, giving it similar gravity, which in turn enables it to retain a thick atmosphere, something the other rocky planets of the Solar System (Mercury and Mars) lack. Unfortunately, the atmosphere of Venus is a little bit too dense for this model to carry any further; Venus is shrouded in a dense cloak of carbon dioxide, resulting in a runaway greenhouse effect and a surface temperature of about 700K (427°C). Despite this apparent hostility, scientists have long speculated that life might exist on Venus, not on its surface, but in the cloud layer between 48 and 60 km above, where Earth-like temperatures and pressures prevail.
Venus has a layer of clouds about 20 km thick, including the 12 km which have Earth-like, temperate conditions. Unfortunately, these clouds are thought to be made not of of water droplets, but concentrated sulphuric acid, with a very low water content, quite unsuitable for any form of life as we understand it.
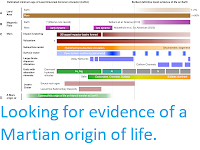
However, 50 years of spectrographic observations of Venus have left a number of unresolved mysteries about the planet's atmosphere.
The most puzzling of these is the presence of water and sulphur dioxide in the atmosphere above the clouds. Water is present throughout the atmosphere of Venus, albeit at very low levels, while sulphur dioxide, which can be measured in parts per million below the clouds, can still be measured in parts per billion above the clouds. Both gasses are thought to be present in the atmosphere of Venus as a result of volcanic emissions, and should in theory be uniformly mixed throughout the atmosphere to an altitude of about 70 km, where they should be destroyed by ultraviolet photodissociation. However, the observed abundances do not support this, suggesting that sulphur dioxide is significantly depleted relative to oxygen in and above the cloud layers.
Previous models have suggested that sulphur dioxide could be photochemically oxidized to sulphur trioxide, which would in turn react with water droplets in the clouds to form sulphuric acid. However, sulphur dioxide is about five times as abundant as water, so if this were happening then all water should be eliminated in the cloud layer, while the sulphur dioxide level should only be reduced by about 20%.
Another persistent mystery is the presence of free oxygen in the clouds of Venus, the formation of which cannot be explained by any current model of the planet's atmosphere and processes. Furthermore, the atmosphere of Venus appears to be in a state of chemical disequilibrium (i.e. it contains gasses that should react with one-another till one or more is eliminated unless they are being constantly being topped up).
Another mystery is the apparent presence of ammonia in Venus's atmosphere. This was first detected by the Russian Venera 8 probe in 1972, although this was initially rejected as an anomaly, but later found again by NASA's Pioneer Venus probe. Pioneer Venus's long-term observation of the clouds of Venus also found further chemical disequilibrium within the cloud layers, as well suggesting that at least the lower layers contained substantial solid material, incompatible with a sulphuric acid composition. Other mysteries uncovered within the cloud layer were the presence of methane and phosphine, as well as an unknown ultraviolet-absorbing molecule.
In a paper published in the Planetary Science Journal on 23 July 2021, Paul Rimmer of the Department of Earth Sciences and Cavendish Laboratory at the University of Cambridge, and the MRC Laboratory of Molecular Biology, Sean Jordan and Tereza Constantinou of the Institute of Astronomy at the University of Cambridge, Peter Woitke of the School of Physics & Astronomy and Centre for Exoplanet Science at the University of St Andrews, Oliver Shorttle of the Department of Earth Sciences and Institute of Astronomy at the University of Cambridge, Richard Hobbs also of the Institute of Astronomy at the University of Cambridge, and Alessia Paschodimas of the Centre for Exoplanet Science and School of Earth and Environmental Sciences at the University of St Andrews, proposed a model in which a base molecule was present in the clouds of Venus, reacting with sulphur dioxide in the atmosphere to form sulphites, and thereby explaining the depletion of sulphur dioxide in the planet's atmosphere.
This reaction would also consume water, but this would be released again as the sulphites broke down in the lower atmosphere, providing a model that would account for the observed levels of water and sulphur dioxide in the atmosphere of Venus. The formation of sulphites would also have a profound impact on the composition, and pH, of the cloud layer. This has previously been assumed to more-or-less pure sulphuric acid, resulting in a pH of about -11, but Rimmer et al.'s model would raise this to somewhere between -1 and 1. This is within the tolerance range of extremophilic Bacteria and Archaeans on Earth, whereas -11 would be completely unsurvivable for any Earthlife-like organism.
In their initial paper, Rimmer et al. used sodium hydroxide as base in their model, not because they thought it was likely to be present, but simply as a standard base with predictable interactions. In a second paper published in the Proceedings of the National Academy of Sciences of the United States of America on 20 December 2021, William Bains of the Department of Earth, Atmospheric, and Planetary Sciences at the Massachusetts Institute of Technology and the School of Physics & Astronomy at Cardiff University, Janusz Petkowski, also of the Department of Earth, Atmospheric, and Planetary Sciences at the Massachusetts Institute of Technology, Paul Rimmer, lead author of the original study, and Sara Seager, again of the Department of Earth, Atmospheric, and Planetary Sciences, and of the Department of Physics and the Department of Aeronautics and Astronautics at the Massachusetts Institute of Technology, suggest that ammonia might be the base present in the clouds of Venus, discuss the implications of that.
The presence of ammonia in the clouds of Venus would result in the observed gas balance, with the upper atmosphere depleted in sulphur dioxide, but still containing water, as well as solid particles being formed in the clouds, and the presence of free oxygen.
In order for ammonia to form in the clouds of Venus, it would be necessary to reduce nitrogen by bonding it to hydrogen, which would require source of electrons as well as a source of hydrogen. Since there is almost no free hydrogen in the clouds, Bains et al. consider water to be the most likely candidate for the hydrogen doner, with electrons being obtained by the oxidation of carbon monoxide, carbonyl sulphide, sulphur dioxide, nitrogen, water, or hydrochloric acid.
Bains et al. reason that the most likely process would be the reaction of nitrogen with water to produce ammonium hydroxide and oxygen, as this requires less water and less energy than other potential reactions, and because it is the only reaction that would directly produce both ammonia and oxygen (which have been observed) without producing other oxidized species (which have not been observed) and would then require more energy to break down releasing oxygen.
The next question Bains et al. address is the rate at which the clouds must be depleting sulphur dioxide from the atmosphere in order to produce the observed levels, assuming that the sulphur dioxide rate is being topped up from below at a steady rate. This leads to the calculation that 100 billion tonnes of ammonia must be produced each year, roughly equivalent to the amount of free oxygen produced by photosynthesis each year. This would also result in about 100 billion tonnes of mass being lost from the bottom of the cloud layer through the precipitation of solid sulphite particles, which is in line with observations.
Any reaction producing ammonia on Venus would require considerable energetic input, leading Bains et al. to look for plausible sources of such energy. Lightning is present on Venus, and is capable of reducing nitrogen to ammonia, but the level of energy available from lightning would be far short of that needed to produce the observed gas ratios, and in any case unlikely to produce free oxygen. Ultraviolet-driven photochemistry can also produce ammonia, but again is most unlikely to do so at the observed levels (although how the presence of large amounts of sulphuric acid would have on this process has never been explored). Similarly, volcanoes can produce ammonia on Earth, but it is highly unlikely that Venusian volcanoes could be producing the observed levels of ammonia.
The ability to harvest chemical energy to reduce nitrogen to ammonia in an oxidising environment is a characteristic of life on Earth, where many Microorganisms live by doing just that, so Bains et al. posit that it is not unreasonable to suggest similar organisms might be carrying out this process in the clouds of Venus. The energy requirements of such a process would be high, and there would need to be clear benefits involved for any organism to do this, but Bains et al. suggest that creating a habitable environment by de-acidifying the cloud droplets would satisfy this requirement.
Bains et al. suggest that the production of the levels of ammonia needed to drive this process is not beyond the plausible range of biological organisms living within the clouds. 100 billion tonnes of ammonia production per year equates to 3.10 billion grams of ammonia per second. On Earth, several species of Cyanobacteria can fix nitrogen into ammonia at rates of 0.000 000 041 g per gram of wet weight per second, which means that 8.1 billion tonnes, wet weight, of these organisms could produce 100 billion tonnes of ammonia per year. Any life in the clouds of Venus is unlikely to closely resemble Earthly Cyanobacteria, but assuming an organism with similar productivity was involved, then it would only represent about 1.5% of the mass of the lowest 5 km of the cloud layer.
Bains et al.'s model provides a better match for the observed abundances of gasses in the atmosphere of Venus, and in particular can account for; (1) the observed disequilibria in the clouds of Venus, (2) the observed presence of oxygen in the clouds, (3) the observed abundance of water in the clouds, (4) the detected ammonia in the clouds, and (5) the observed abundance profile of sulphur dioxide in and above the cloud layer.
Oxygen was detected in the clouds of Venus by the Pioneer Venus and Venera 14 probes, but this was rejected at the time due to the implied disequilibria that would have to exist in Venus's atmosphere. However, since that time is has become overwhelmingly clear that something is causing a disequilibrium in the atmosphere of Venus, and it is therefore reasonable to assume that oxygen is present. Bains et al.'s model predicts the presence of oxygen at levels at only one twentieth of the levels observed by Pioneer Venus and Venera 14 (although it does not rule out the production of further oxygen by other biological processes), but at a far higher level than any other model currently available.
Ammonia was also observed in the clouds of Venus by the Pioneer Venus and Venera 8, but this finding was also rejected at the time, as it was assumed the clouds were made up of droplets of concentrated sulphuric acid, and environment in which ammonia could not survive. Bains et al.'s model produces a much less acidic cloud layer, potentially with a pH above zero, within which ammonia could potentially exist.
Bains et al.'s model predicts the precipitation of ammonium sulphate and ammonium sulphite beneath the cloud layer, forming droplets that would fall until they are evaporated by the rising temperature of the atmosphere. This would result in the thermal decomposition of these molecules, which would release free ammonia, which would be oxidised to form nitrous oxides, something else detected by Pioneer Venus.
This model would also result in larger, probably non-spherical, droplets within the lower cloud layer, which would present a potential home for any biological organism. This is consistent with observations of the cloud layers on Venus, which again cannot easily be explained by any non-biological process.
Bain's et al.'s model can also explain the 'stagnant haze' that has been observed beneath the cloud layer on Venus. This haze extends downwards from the cloud base at 47 km above the ground, to an altitude of about 30 km, and its composition is unclear. The temperature of this haze layer is about 100°C at its upper extent and about 200°C at its base. This would be consistent with sulphate and sulphite salt particles falling from liquid droplets that evaporate at about 100°C, and which themselves decompose at temperatures of 200°C. This would also result in the formation of hydrogen sulphide in the layer beneath the clouds, something else which has been tentatively identified in the data from the Venera 14 and Pioneer Venus probes.
This study provides a new perspective on the habitability of the clouds of Venus. These clouds have previously been viewed as made up entirely of sulphuric acid, creating an extremely acidic and extremely dry environment, something deeply hostile to even the most tolerant forms of life. Bains et al.'s model shows how living organisms could inhabit a layer in the lower parts of these clouds, maintaining a much less acidic environment, with larger semisolid ammonium sulphite and sulphate particles through the production of ammonium.
The predicted pH within this layer, -1 to 1, is within the tolerance levels of some Extremophilic micro-organisms on Earth, and the temperature range, 60-80°C, is that which would be considered optimal for many such organisms. Furthermore, many micro-organisms which we would not considered extremophilic can produce ammonia as a defence against acidic conditions (including some pathogens, such as Mycobacterium tuberculosis and Candida albicans, which use this ability to neutralise the acids in phagosomes produced by our bodies as a defence against them).
Bains et al. note that there are still problems with their model. Most notably, the model only predicts a relative humidity of 0.02% in the lower cloud layer. This is 50 times drier than the lowest conditions that can be endured by the most drought-tolerant organisms on Earth (spores, and aestivating stages of Tardigrades and some micro-organisms can survive such conditions, but are not biochemically active). However, the levels of water reported in studies of the cloud layer is variable, which may mean that the distribution of water within these clouds is variable, with both arid and humid regions, allowing for the presence of active life.
The origin of life on Venus is an interesting question. Some models of Venus's history suggest that the young planet was much more hospitable, with a temperate climate and liquid water on its surface, with hostile conditions developing later due to a runaway greenhouse effect. Under such a model, life could have originated on the surface of Venus, and subsequently migrated into the clouds (this is not unreasonable as, although it is not obvious, there is quite a bit of life in the Earth's upper atmosphere).
See also...


Follow Sciency Thoughts on Facebook.
Follow Sciency Thoughts on Twitter.