Dextrally coiled Pelagiellids, globally distributed in Early to Mid-Cambrian assemblages of small shelly fossils, are among the best known of the early Conchiferans (one of two subphyla of the Molluscs, which includes the Monoplacophorans, Bivalves, Scaphopods, Cephalopods, and Gastropods). Their tiny shells (mostly 1–2 mm across, but Pelagiella atlantoides reached 9 mm) expand asymmetrically away from the plane of the first whorl, down the spiral axis, then back upward to establish an adult form that is almost planispiral, with a depressed apex. They have been treated implicitly or explicitly as stem group Conchiferans, as untorted Helcionelloids (an extinct branch of the Conchiferans known from the Cambrian and Early Ordovician), as Monoplacophorans, and explicitly as Gastropods presumed to have undergone torsion during their development (torsion, rotation of the visceral mass by 180° relative to the head and foot during larval development, is considered to be a key feature of Gastropods). Recently, together with the Aldanellids, they have been linked erroneously to the Hyolitha.
In a paper published in the journal Palaeontology on 16 March 2020, Roger Thomas of the Department of Earth & Environment at Franklin & Marshall College, Bruce Runnagar of the Department of Earth, Planetary, & Space Sciences and Molecular Biology Institute at the University of California, Los Angeles, and Kerry Matt of Lancaster, Pennsylvania, report the discovery, in the Kinzers Formation of south-eastern Pennsylvania, of numerous specimens of Pelagiella exigua with associated impressions of two clusters of chaetae (chinious bristles).
Scanning electron microscope images illustrating shell forms of Pelagiella spp. (A) Steinkerns (natural internal moulds) of Pelagiella sp., from limestone at Thomasville, Pennsylvania, assigned to the basal Kinzers Formation or to the uppermost Vintage Formation; PMU 25156, 25157, apical and apertural views; comparable in size and shape with shells of Pelagiella exigua flattened in shale of the Emigsville Member, with which they may be conspecific. (B) Shells of Pelagiella subangulata, NMVP 120790, oblique right anterior dorsal and oblique left posterior dorsal views, and SAMP 29038, apertural view, illustrating extreme development of the protruding ‘auricle’ on the left, abapical side of the aperture; Parara Limestone, Cambrian Stage 3, Ardrossan, South Australia. Scale bar represents about 0.5 mm. Thomas et al. (2020).
The occurrence of a pair of chaeta-bearing appendages, inferred to have extended from the anterior body wall or foot of the animal, is unique among Molluscs, living and extinct. Moreover, chitinous chaetae, characteristic of Annelids and Brachiopods, have been cited as a homologous feature of Lophotrochozoans, so the occurrence in the fossil record of an early Conchiferan with chaetae has phylogenetic implications. Thomas et al. document the new material in detail, assessing its implications for the anatomy of Pelagiella, its likely mode of life, and its potential role in the emergence of the Gastropoda.
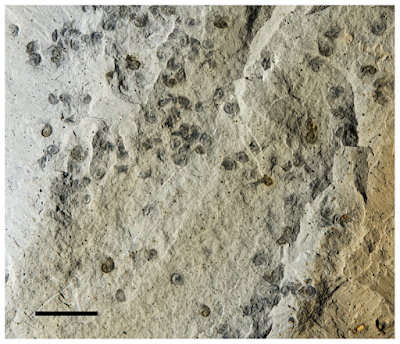
Contrast-enhanced photographs of fossil shells of Pelagiella exigua with chaetae preserved. (A) Death assemblage, about 20 shells lying at various angles to the surface of a bedding plane; shells empty at time of burial (grey, white arrow) are completely flattened; those partially filled by sediment and organic material (brown, iron oxides, black arrow) include partial internal moulds; note several clusters of chaetae, USNM 617253p. (B) Partial internal mould of a shell with two sets of asymmetrically disposed anterior–lateral chaetae, USNM 617253cp, #12. (C) A chaetal cluster of Pelagiella exigua; impressions of chaetae, preserved in three dimensions in fine siltstone, converging downward to a common point of attachment of the bundle in the body wall or foot; when extended, the chaetae thus formed a broad, funnel-shaped hemiconical array, USNM 617254p. Scale bars represent: 2 mm (A); 0.5 mm (B), (C). Thomas et al. (2020).
Lophotrochozoans are Protostomes, united as a clade by the results of molecular phylogenetic analyses. Their shared morphological characters are limited mainly to features of their embryos and larvae. Phylogenetic studies recognise a subsidiary Trochozoan clade, including Annelids, Brachiopods and Molluscs, in which chitinous chaetae appear to be a common ancestral character. In the Mollusca, chaetae may have been lost among taxa assigned to the Aculifera (the other subphylum of the Molluscs, which includes the Aplacophorans and Polyplacophorans) and, once or repeatedly, in diversification of the Conchifera during the Cambrian Explosion (535–515 million years ago). Alternatively, chaetae may have been retained by the Aculiferans, if the chitinous cores of their calcareous sclerites are indeed homologous with chaetae, but generally not in the Conchifera.
Relationships among the Molluscan classes have yet to be fully resolved, probably in part because their evolutionary radiation was so rapid. The advent of molecular phylogenetics raised the hope that these long-standing difficulties would be overcome. Recently, a near-consensus emerged on the view that the Molluscs evolved to constitute two major clades, the Aculifera and the Conchifera. Subsequently, very different phylogenetic relationships have been inferred from comparison of numerous published Molluscan trees, and from time-calibrated phylogenetic analysis of a more broadly representative Molluscan data set that posits Gastropods as the sister group of a clade including Bivalves and Serialia (Monoplacophora and Polyplacophora). From a different perspective, privileging the hypothesis that a U-shaped gut may be homologous among Lophotrochozoans have revived the link between Gastropods and Cephalopods, as Cyrtosoma.
Thomas et al. have framed their assessment of Pelagiella in terms of the Aculifera–Conchifera paradigm, which has been widely adopted. If this view is largely correct, the diversification of Molluscan taxa reflects the adoption of markedly different modes of skeletal evolution and development characterising the two major clades. Among early fossil Molluscs, stem group Aculiferans, almost certainly including Calvapilosa and more controversially Halkieria and Orthrozanclus, evolved scleritomes consisting of discrete, more or less varied and integrated elements, secreted across a broad mantle field. In contrast, mostly diminutive cap or cone shaped small shelly fossils, including Pelagiella, represent continuous, logarithmically expanding, univalve or bivalve skeletons of organisms from which the familiar shell bearing classes of the Conchifera are inferred to have evolved. Thomas et al. show that Pelagiella was both an innovator in the differentiation of these classes and, given its chaetae, the bearer of a deeply rooted feature of its Lophotrochozoan ancestry.
Olenellid Trilobites and associated early Cambrian shelly fossils have been known from the Kinzers Formation of Lancaster and York counties, Pennsylvania, USA, for well over 100 years. Discovery of antennae attached to the type specimen of Olenellus getzi and later recovery of Algae, parts of lightly sclerotised exoskeletons of Arthropods (notably Anomalocaris and Tuzoia) and other soft-bodied organisms, as well as novel Echinoderms, confirmed the modest but significant role of Kinzers Lagerstätten and associated faunas in documenting the sequential development of the ‘Cambrian Explosion’ of Metazoans.
The Kinzers Formation accumulated during the second cycle of early Cambrian transgression of the Iapetus Ocean onto Laurentia. Paralleling the regional strike of the Appalachians, it consists largely of limestone, inferred from widespread submarine debris flows to have accumulated in outer shelf and deeper water periplatform environments. At its base, a stratigraphically distinctive shale defined as the Emigsville Member persists for 40 km along strike. It is in this shale that the relatively diverse fauna associated with the Kinzers Formation almost entirely occurs.
Geology of Lancaster County, showing outcrop trend of the Kinzers Formation along strike of the Appalachian fold belt in south-east Pennsylvania. The Emigsville Member (shale and fine siltstone) occurs at the base of the Kinzers Formation on both sides of the Susquehanna River (south-west border of the county), from Thomasville, west of York (22 km off this map to the west), to Kinzers, 21 km east of Lancaster (city). Inset map shows sites in Manheim Township (SB) and East Petersburg (22L) where Pelagiella exigua has been found with clusters of chaetae preserved. Localities in Lancaster County yielding fossils exhibiting special preservation are all within 6–8 km west, north-west and north of the city of Lancaster. Thomas et al. (2020).
Three facies of the Emigsville shale, broadly distributed along a north-west–south-east onshore–offshore gradient of carbonate/clastic accumulation and inferred depth of the seafloor, have been differentiated into: (1) impure carbonate; (2) massive pelitic; and (3) fine pelitic facies. The first consists primarily of relatively thickly bedded calcareous pelite and siltstone, with abundant skeletal allochems (commonly leached) and burrows recording extensive bioturbation. The latter two facies are differentiated by greater bed thickness and more silt in facies (2), compared with the predominance of fine lamination, limited bioturbation, and common occurrence of diagenetic euhedral pyrite in facies (3). The former presence of pyrite, now oxidized as goethite or entirely leached away, is commonly represented by cubic voids, typically 60–200 lm on a side. This implies preservation of organic matter and reducing conditions within the sediment, but not necessarily on the surface of the sea floor. Rhythmically bedded sediments of facies (3) have been further differentiated as fine and shelly tempestite taphofacies, set apart from occasional mat-grounds.
Deposition of the Kinzers Formation as a whole has been characterised as basinal. However, neither the fine clastic sediments interpreted as tempestites, nor the diverse shelly faunas preserved at some localities, suggest that the basal Emigsville shale was deposited off the continental shelf.
Kinzers Lagerstätten, where lightly sclerotised and soft parts are preserved, occur in the pelitic facies (2) and (3). No one factor facilitating special preservation has left a definitive signature on these facies. Authigenic pyrite, fine-scale graded beds, and trilobites preserved in full articulation at some horizons implicate anoxia, burial by sudden influxes of sediment, or possibly local, ephemeral excursions of salinity or magnesium enriched brine. However, the recurrence of distinctive associations of bottom dwelling organisms in Emigsville faunas and the lack of evidence of long-distance transport reflect an environment that was not generally inhospitable to life on the sea floor.
Pelagiella, including the new material described by Thomas et al., most commonly occurs in the Fine Pelitic facies of the Emigsville shale, in association with Brachiopods and Hyoliths, but not together with Algae or taxa dependent on special preservation.
Modern systematic work has enhanced our knowledge of the Emigsville fauna. Nonetheless, the age of the Kinzers Formation is still not well constrained, being determined by its fauna to lie within the classic Olenellus–Bonnia Zone. Since strata well above and below the Emigsville Member also yield Olenellus, it may be inferred that the age of the Emigsville fauna is late Dyeran (Cambrian Series 2, Stage 4, or roughly 512-million-years-old). Hence the age of this fauna lies between those of strata yielding the Chenjiang fauna in China (Series 2, Stage 3, Qiongzhusian or about 518-million-years-old) and the Burgess Shale in Canada (Series 3, Drumian, about 505-million-years-old). The Emigsville shale shares both its preservation of soft-bodied fossils and many characteristic taxa with these better known biotas.
Specimens of shale and very fine siltstone bearing Pelagiella exigua with associated impressions of paired clusters of chaetae were obtained from a temporary excavation in the Emigsville Member of the Kinzers Formation, at the intersection of Settlers Bend and Buch Avenue, in Manheim Township, 6 km north of Lancaster, Pennsylvania. Over 100 recognizable shells of Pelagiella exigua occur on a single 10 9 8 cm part-and-counterpart slab (USNM 617253p, 617253cp). A smaller piece of shale (USNM 617254p, cp, part and counterpart) preserves a coquina of shells, now external and internal moulds, The shells were studied by light microscopy, without special preparation. Photographs of the slab and counterpart were taken with a Nikon D750 and of the coquina with a Leica M 80 HD microscope and camera at Franklin & Marshall College. High-resolution photographs of individual shells and their chaetae were taken using a Leica MZ16 microscope at Yale University. Scanning electron microscope images of chaetae aligned in the form of a three-dimensional cluster were acquired by a Philips XL 30 environmental scanning electron microscope, also at Yale University. The specimens have been deposited, with permission of the owners of the property from which they came, in the collection of the Department of Paleobiology at the US National Museum of Natural History, in Washington DC.
Bedding plane surface showing a broad scatter of shells of Pelagiella exigua. Over 100 shells (some beyond field of view) occur here in varied states of preservation. They range from partially lithified internal moulds to flattened ‘ghosts’ of shells, all but lost to solution and diagenesis, USNM 617253p. Scale bar represents 1 cm. Thomas et al. (2020).
The newly described specimens of Pelagiella exigua occur in two distinct associations. In one case, more than 100 shells are scattered across a single bedding plane, on part and counterpart surfaces of a 10 9 8 cm slab of shale (USNM 617253p). The shells are similar in size, a little more or less than 2 mm in diameter. The shells were compacted during early diagenesis and subsequently dissolved. Their preservation varies from partial internal moulds with some three-dimensional relief to flattened composite moulds. These represent two to four originally separated inner and outer surfaces, in a plane where all preserved features are superimposed. Some are no more than flattened ‘ghosts’ representing little more than outline shapes of the shells.
Colour and contrast enhanced photograph of a coquina of shells of Pelagiella exigua, preserved as internal and external moulds. Numerous clusters of extended chaetae (arrows) are also preserved, USNM 617254p. Scale bar represents 2 mm. Thomas et al. (2020).
Previous research has likewise observed partial steinkerns inside shells of Pelagiella from the Emigsville Member of the Kinzers Formation. Energy dispersive spectroscopy has shown them to consist largely of pyrite (since oxidized to goethite), phosphate and aluminosilicates. He inferred this mineralization to represent partial replacement of soft tissues, mediated by microbial action. Differences in preservation among our specimens, in close proximity to one another, are here inferred to record variation in the extent to which decaying tissue promoted early mineralisation within the shells.
Shells of Pelagiella exigua with chaetae in different states of preservation, buried at various angles to the surface of the sea floor. (A) Anterior view of a compressed internal mould, with clusters of chaetae fully extended from the auricle (flange on the right, anatomically the left side of body) and from the anterior–lateral margin of the flat, apical side of shell, USNM 617253p, #29. (B) Umbilical surfaces of two shells, one with right-side cluster of chaetae extended, left-side cluster apposed, USNM 617253cp, #5, #6. (C) Two shells less compressed due to early lithification of partial steinkerns; left, umbilical view with left-hand cluster of chaetae partly apposed; right, apical view, with right-hand cluster of chaetae fully extended, USNM 617253cp, #25, #26. (D) Umbilical, under surface of shell with right-side chaetae extended and left-side cluster tightly apposed but not withdrawn into shell, USNM 617253cp, #8. (E) Anterior–apical view of shell with a substantial ferruginous steinkern and both sets of chaetae extended, USNM 617253p, #62. (F) Left cluster of chaetae emerging from auricle of a shell crushed from posterior to anterior; right cluster partially extended, tightly apposed to the right, USNM 617253p, #59. (G) Oblique posterior view of a flattened shell with left cluster of chaetae emerging anterior of auricle and right cluster extended anterior of the umbilicus, USNM 617253p, #28. (H) Extended chaetae showing variable, slight ferruginous mineralisation, USNM 617253cp, #5 . (I) Ventral view showing anterior margin of the shell, chaetae emerging from auricle. (anatomical left) on the right and anterior of umbilicus on left, USNM 617253p, #91. Scale bars represent: 1 mm (A)–(G), (I); 0.5 mm (H). Thomas et al. (2020).
The shells occur in a variety of orientations. On the counterpart surface, 11 of them have apertures clearly facing to the right; in 20 cases they spiral to the left. Many shells show by their roughly triangular cross-sections that they were embedded at an angle in very soft sediment. Most of the animals were lying on their sides. These observations imply that the organisms were buried suddenly and not in a consistent in life orientation.
More than a third of the shells on the slab are accompanied by impressions of one or two clusters of fine, straight or gently curved bristles. These extend 0.5–0.8 mm outward from what are inferred to have been the anterior left lateral and the right anterior margins of the shell, The bristles in some clusters are tightly apposed, but in most cases they splay as broad fans, flattened in two dimensions. From their clustered associations, their dimensions, and the modest flexibility indicated by their variable curvature (conjunct within a cluster), we infer these bristles to have been chaetae.
Preservation of these impressions, some of which are stained by iron oxides, may have been enabled by inhibition of Bacterial decomposition by clay, or by rapid preservation of silica-cement, if the high silica saturation state of the Ediacaran ocean persisted through the early Cambrian. Subsequently, the chaetae may have been filled or replaced by pyrite framboids during diagenesis, like those of Polychaete Annelids in the Burgess Shale.
The smaller piece of shale (USNM 617254p, cp), represents a different association. It displays a dense aggregate of moulds of shells, swept together at all angles and less flattened. Many of these shells are also accompanied by impressions of chaetae. Two of these clusters of chaetae are preserved in three-dimensions, showing their arcuate proximal attachment around a semicircular node at the base.
Cluster of chaetae of Pelagiella exigua converging on basal node of attachment of the array, USNM 617254p. (A)–(C) Scanning electron microscope images at successively higher resolutions show preservation of chaetae as grooves (i.e. moulds) in very fine siltstone and clay from which the original organic material has been dissolved away. (D) photograph of the entire chaetal organ showing the area enlarged as (A)–(C) outlined in white. Scale bars represent: 100 μm (A); 50 μm (B); 20 μm (C); 0.5 mm (D). Jakob Vinther in Thomas et al. (2020).
The preservation of monospecific assemblages of similarly sized shells of Pelagiella exigua, many with associated impressions of chaetae, implies that the organisms were alive or very recently dead when they were buried. These Animals may have been swept up from the surface of Algal/Bacterial mats, they may have fallen from life on the surfaces of Algal fronds, or if they were pelagic they could simply have sunk through the water column in conjunction with the event that killed them. This last alternative is unlikely, given both the size and shell thickness of these organisms and the global absence of Pelagiella from pelagic faunas in deep ocean sedimentary rocks.
Faced with adverse conditions, animals with protective shells generally withdraw into them. Some environmental insults cause organs to contract violently and become contorted. However, these organisms appear have been induced to relax following their demise, causing their chaetae to be extended to varying degrees postmortem. Asphyxia resulting from hypoxia or high concentrations of magnesium ions can bring about this kind of relaxation, so either of these factors could have been involved here.
The impressions of bristles emerging in clusters from the apertures of shells of Pelagiella exigua, interpreted here as chaetae, rarely cross and they do not branch. The chaetae are 5–10 μm wide and up to 1.3 mm long. As many as 50 chaetae occur in each cluster. The clusters emerge in various directions, depending partly on the orientation of shells relative to their flattening in the shale. Generally, the cluster on the apical side of the shell (anatomical right in Thomas et al.'s new reconstruction) emerges perpendicular to the inferred margin of the aperture, whereas that on the umbilical side (anatomical left) radiates obliquely from its anterior midsection.
For example, chaetae aligned perpendicular to the periphery of the whorl project from underneath the laterally compressed shell, whereas chaetae on the left-hand side of the animal appear where they emerged, preserved in the plane of compression. The orientation of chaetae on the umbilical side of this individual, parallel to the surface of the shell, is inferred to be a result of rotation during compaction. In some specimens, the clusters of chaetae are more or less tightly apposed, showing that they were extensible and could be retracted, although possibly not all the way back into the shell.
If Pelagiella exigua was benthic and lived with its tangential aperture parallel to the substrate, like modern Gastropods with capacious apertures, the chaetae must have projected outward rather than downward from the visceral mass or foot. In flattened specimens with the best organized arrays, the chaetae of each cluster appear to converge toward points well within the interior of the body whorl. Numerous specimens with two discrete clusters of chaetae still in place suggest singular sites of attachment, on either side of the body. A few in situ clusters are somewhat disordered, with chaetae misaligned and crossing one another. These are thought to reflect post-mortem disaggregation.
A remarkably preserved, isolated three-dimensional array of chaetae documents the proximal configuration of the clusters, otherwise hidden within the compressed shells. The chaetae converge on a basal node, potentially a mould replacing the muscle mass by which they were articulated. The funnel-shape of this disjunct cluster and its basal node imply that in life the chaetae were extended as shallow hemiconical arrays, rather than as planar fans.
The states of preservation of the chaetae show them to have been stiff but slightly flexible in life. The consistent form, disposition and orientations of the clusters of chaetae with respect to the shells indicate that they were features of the shell maker, not those of an adventitious occupant of dead shells. These structures were comparable in form and inferred stiffness/flexibility with chitinous chaetae of living Brachiopods and many Polychaete Annelids.
Given these comparisons, the chaetae of Pelagiella exigua might be expected to have been deployed along the mantle margin, as in adult Brachiopods, or anchored in follicles of parapodia-like appendages, as in Polychaetes. Insertion along the mantle margin is ruled out by the configuration of the chaetae as clusters that converge toward points of attachment well inside the shell. Furthermore, significant masses of protractor and retractor muscles would have been required, as in Polychaete parapodia, to operate numerous chaetae as components of an array that was large in size relative to that of the Animal.
The clusters of chaetae might have been deployed by parapodia-like appendages analogous to the epipodial tentacles of Vetigastropods. More probably, given their inferred points of convergence, they were attached further back on the left and right sides of the foot. Protraction of the foot might have allowed the arrays to protrude a considerable distance beyond the shell. Retraction could have withdrawn them well into the mantle cavity, forming a fringe of chaetae extending around much of the shell’s aperture. On the other hand, the asymmetric alignment of the chaetal appendages relative to the anterior–posterior axis of the Animal suggests that they could have been independently protruded and retracted.
Taken together, these observations and inferences lead to the conclusion that Pelagiella exigua was equipped with a pair of anterior–lateral, muscular, parapodia-like appendages, arising from the body wall or the foot, each bearing a cluster of chaetae that could be extended as a hemiconical array and to some degree retracted.
Chitinous chaetae, canonical features of Annelids and Brachiopods, are not known as such in other living Lophotrochozoans. However, Mopaliid Chitons, juvenile Octopods and Bryozoans employ chitinous materials with ultrastructural similarities to those of chaetae and chitin is an essential component of the organic matrix of Mollusc and Brachiopod shells. Well-preserved fossil chaetae are known from Cambrian Polychaete Annelids and Brachiopods. Among small carbonaceous fossils from the late Cambrian of Baltica, isolated strap-shaped chaetae, with bifid and serrated tips, like the brush chaetae of modern Annelids have been recognised. The chaetae of Pelagiella exigua are similar in size to these fossils, but dissimilar in form, comparable to the ‘indeterminate spinose elements’ reported in the same assemblages.
The capacity to synthesize chitin predates the common ancestor of Animals and Fungi, and chitin synthases belonging to a monophyletic gene family are widely distributed among the Lophotrochozoan phyla. The genome of the Limpet Lottia gigantea codes for nine chitin synthases belonging to four paralogous groups that also occur in Annelids. Bivalves, a Chiton and a Brachiopod are also known to express some members of these groups. Most of these chitinases have a common N-terminal myosin motor domain that interacts with the actin cytoskeleton to deliver the enzyme to sites of secretion. In both Annelids and Brachiopods, synthesis occurs in chaetoblasts of the chaetal follicles, development being controlled by the expression of particular Hox and homeodomain genes. Thus, the genetic underpinnings required for chitin synthesis and chaetal construction were present in the common ancestor of Annelids, Brachiopods and Molluscs.
Phylogenetic tree representing likely relationships of some Lophotrochozoan higher taxa and the inheritance pathways for the subsets that retain or have lost (black) the ability to secrete chitinous microfibrillar tubules (CMTs), the structural component of chaetae (LCSs) and other similarly derived products. Coloured rectangular bars represent crown groups (orange, Brachiozoa; yellow, Bryozoa; green, Annelida; blue, Mollusca); mineral names refer to the primary skeletal biomineral of each clade. Stem taxa in addition to Pelagiella (Pe) that have preserved evidence of chaetae or chitinous microfibrillar tubules are indicated by: (C) Canadia; (M) Mickwitzia; (N) Micromitra and Paterina; (O) Oymurania; (P) Plaesiomys; and (W) Wiwaxia. Other abbreviation: op, operculum. The unresolved quadrichotomy linking higher level clades reflects uncertain relationships amongst them. Thomas et al. (2020).
Up to now, only two of these phyla have been known to express the capacity to construct chaetae as such. In the Mollusca, this capacity would appear also to have been retained if the uncalcified dermal sclerites of the putative stem group Mollusc Wiwaxia are indeed homologous with chaetae and if the calcareous sclerites of the Aculiferans are also homologous with chaetae. Among the Conchiferans, the corresponding genes may be employed mainly in producing chitin for the organic matrix of calcareous shells and Gastropod opercula. These inferences are all contested. The issue turns on whether structures composed of chitin that have evolved in different anatomical settings are regarded as being homologous, or as being independent, convergent applications of the same molecular and tissue-building toolkit. In fact, processes that are homologous at one level of pattern formation may be expressed independently at different levels or sites of anatomical deployment. Here, this issue does not arise, as we present direct evidence of the occurrence of chaetae as such in the stem group Gastropod Pelagiella.
Chaetae may have sensory, locomotory, feeding or protective functions. Pelagiella exigua had two sets of chaetae, long, slender and apparently flexible, like capillary chaetae of Annelids, but stiff enough to have been articulated as cohesive parts of the appendages to which they were attached. Chaetae employed by Annelids in swimming, often jointed and with lateral flanges, are typically deployed along the length of the body. The two arcuate chaetal arrays of Pelagiella exigua appear neither to be appropriate in form nor were they anatomically well deployed to serve this purpose.
Given the widely gaping, angular aperture of its shell, it is unlikely that Pelagiella had an operculum. Consequently, Pelagiella exigua might have benefited from a protective screen, excluding interlopers and coarse sediment, as in some Brachiopods. But the form, flexibility and occurrence in clusters of the chaetae indicates an active function, not the passive role of a rigid fence or grating. Tactile sensation, observed as a function of chitinous hairs of living Chitons (e.g. Mopalia muscosa), has been inferred for the radial arrays of exceptionally long chaetae of the Cambrian Brachiopod Micromitra burgessensis. This purpose seems less likely to have been well served by the two integrated clusters of chaetae wielded by Pelagiella exigua than it would have been by more broadly distributed exploratory sensors.
Hence, in part by exclusion, but directly on account of the fact that the chaetal arrays appear to have faced outward and forward, it seems most likely that Pelagiella exigua employed its chaetal appendages primarily in food gathering. If these Snails lived on the surface of the sea floor or in large numbers on seaweed, both consistent with their palaeoecology and modes of preservation, they may have deployed their chaetae like the cephalic cages of Flabelligerid Annelids to trap and harvest small organisms and flocculent or floating detritus. The extended chaetal arrays of Pelagiella exigua are remarkably similar in form to arrays of hairs borne by the second maxillae of presentday Calanoid Copepods. Although larger (x5, linear dimensions), the chaetal arrays of Pelagiella exigua could likewise have operated as leaky sieves, in ‘fling and squeeze’ trapping of food particles. Alternatively, in environments featuring high concentrations of dissolved nutrients and intermittent deprivation of oxygen, the large surfaces of the chaetal arrays might even have been employed in Bacterial farming, like that increasingly recognized in the deep ocean today. Finally, if Pelagiella exigua were mobile on the seafloor, its chaetal arrays could have served as whisk-brooms, stirring up detritus and meiofaunal organisms to be sorted by epipodial or cephalic tentacles before being passed to the mouth. This mode of feeding is common among Annelids, and not dissimilar to that of Gastropods adapted to suspension or selective deposit feeding.
There has never been any real doubt that Pelagiella was a Mollusc. But disagreement persists over whether it was a Helcionelloid expressing partial torsion, hence a stem group Gastropod, a divergent, untorted Paragastropod, an untorted, endogastric relative of Helcionelloids, or a fully torted stem, or even crown group, Gastropod. Resolution of this issue depends on indirect evidence of the disposition of soft parts of the Animal that are not preserved and their orientation in relation to its shell.
Initially, real but misleading points of comparison led George Frederick Matthew to interpret the type species on which he defined Pelagiella in 1895 as a swimming Heteropod. The shell of Pelagiella atlantoides has a widely gaping aperture that is unusually thickened (‘gerontically’) around part of its margin. From these features of derived and geologically much younger pelagic Gastropods, Pelagiella gained its inappropriate name.
In 1952 James Brookes Knight sought to locate the origin of torsion among Cambrian Helcionelloids. Acknowledging limited evidence, he reconstructed the anatomy of Coreospira and Oelandia as fully torted Gastropods. But he remained uncertain to the end that Pelagiella was a Gastropod, citing in particular the unusual shape and thickened margin of its aperture. In his view ‘Pelagiella and its allies’ constituted a distinct, untorted lineage established just prior to the emergence of the Gastropoda.
In the 1970s Bruce Runnager and John Pojeta rejected this analysis, interpreting all bilaterally symmetrical Helcionelloids as untorted Monoplacophorans. They supposed, citing consistent dextral coiling of their helical shells, that Aldanella and Pelagiella were among the earliest known Gastropods. Later, Runnegar recognised left and right muscle scars, offset by about 10° along the anterior–posterior axis of a remarkably well preserved steinkern of Pelagiella atlantoides. From associated ridges interpreted as traces of mantle attachment to the shell, he inferred that space occupied by the mantle cavity was incompatible with 180° of torsion. Hence, he treated Pelagiella as a Helcionelloid that was slightly torted.
Steinkern of Pelagiella atlantoides showing muscle scars and arcuate ridges, reflecting grooves on the interior surface of the shell, inferred to have been associated with attachment of gills to the mantle. (A) Right, apical side of shell with the apex depressed, showing a single groove extending anteriorly from the vicinity of the pedal retractor muscle scar (arrow). (B) Left, umbilical side of the shell, showing grooves extending both anteriorly and posteriorly away from the pedal muscle scar (arrow). USNM 298724, Hanford Brook Formation, Protolenus elegans Zone, basal Cambrian Stage 5, St John, New Brunswick, Canada. Scale bars represent 2.0 mm. Jakob Vinther in Thomas et al. (2020).
Also in the 1970s Robert Linsley argued that the shape of the aperture of Pelagiella, elongated perpendicular to the coiling axis of its shell is uncharacteristic of Gastropods. In 1984 Linsley and William Kier suggested that this implied a body plan calling for assignment of the pelagiellids to an extinct class of early untorted Molluscs, which they established as the Paragastropoda. In 1991 John Peel also doubted that Pelagiella was a Gastropod, despite supposing that its shell coiling was endogastric. He assigned it, with Aldanella, to an early branch off the Conchiferan stem, close to the Helcionelloida.
A cladistic analysis of early Palaeozoic Gastropods based entirely on external shell characters (i.e. without reference to infrequently preserved muscle attachment scars), carried out by Peter Wagner, endorsed the suspicion that the taxon Paragastropoda is not monophyletic. In this analysis, pelagiellids appeared as the sister group of Onychochiloid Gastropods, with which they were supposed by Jerzy Dzik to share similar protoconchs. These taxa were next most closely linked, as suggested by various previous authors to the Helcionelloids. Wagner considered all these taxa to have been untorted, conventionally assuming the origin of torsion to have coincided with the emergence of an apertural sinus or slit required to accommodate the sanitary requirements of a post-torsional mantle cavity.
Alternative reconstructions of Pelagiella. (A) As a Helcionelloid ‘Monoplacophoran’, slightly torted with the two pedal muscle scars offset by 10°, the shell’s apex oriented anteriorly, and paired gills in the posterior mantle cavity. (B) As a Helcionelloid Gastropod, with the shell’s apex oriented posteriorly, a reduced right gill, and an inhalant current drawn in through a strong deflection in the aperture (auricle) as seen in Pelagiella subangulata; the posterior organ shown represents a large mass of gonad, inferred to have been present by comparison with the anatomy of living Vetigastropods. (C) Thomas et al.'s interpretation, as a stem group Gastropod with paired chaeta-bearing appendages and asymmetrically developed anterior gills; these are inferred to have been attached to the mantle where it was anchored in fine grooves on the interior shell surface; the asymmetric ctenidia and left/right offset of pedal muscles are consistent with full torsion and asymmetry of helical shell coiling; the posterior organ shown here represents digestive gland, based on phosphatised branching tubules, mainly to the right and behind the gut, preserved in Costipelagiella sp. Thomas et al. (2020).
In contrast, Russian scholars now assign Pelagiella and all other Helcionelloids to the Gastropod subclass Archaeobranchia. They suppose that all these early Molluscs, most of them bilaterally symmetrical and some with uniquely specialized shell forms (e.g. Latouchella, Yochelcionella) underwent full developmental torsion. This view is based largely on presumed endogastric coiling, the disposition of retractor muscle scars inferred from microstructural fabrics observed in some taxa, and an anterior mantle cavity based on reconstructions of the circulation of respiratory currents.
More is known about the soft-parts of Pelagiella than most fossil gastropods without living counterparts. Thomas et al.'s discovery of two sets of chitinous chaetae adds to this body of evidence, which may be used to test previous reconstructions of its anatomy and to prompt consideration of a new one.
Patches of polygonal impressions and other features interpreted as muscle scars have been reported from phosphatic internal moulds of several species of Pelagiella. Some of these reports may represent evidence of muscle attachment, but they are all equivocal. One previous study figured an internal mould, designated as Pelagiella sp. from Morocco, bearing a kidney-shaped feature on its umbilical surface. This was interpreted as the attachment scar for a large columellar retractor muscle, comparable to that of the Abalone, Haliotis. However, this feature lacks evidence of a muscle track, scalloped edges, or imprints of myostracal prisms that would confirm it to be a muscle attachment scar.
Another study inferred the occurrence of a columellar muscle in Aldanella. This study reported a spiral groove on the apical surface and a weakly defined spiral ridge on the umbilical surface of a steinkern of Aldanella kunda from Cambrian Series 1 strata in Estonia. These features appear to be comparable to a pair of deeply pitted circum-umbilical grooves on the apical and umbilical margins of steinkerns of two species of Pelagiella, interpreted as impressions of narrow ridges serving as sites of muscle attachment. These features are better placed, adjacent to the axis of the shell, to be interpreted as traces of attachment for columellar muscles.
Evidence of a single pair of retractor muscles preserved on a topotype steinkern of Pelagiella atlantoides is less ambiguous. The two scars have the elevation and sharp edges of muscle scars observed on internal moulds of fossil Bivalves where their interpretation is unequivocal. Associated with these scars on both sides of the steinkern are fine, gracefully curved ridges, reflecting grooves on the interior surface of the shell. In each case these turn inward toward the muscle scars, as would be expected if they represent lines of attachment of the mantle to the shell, at its junction with the visceral mass. Since pedal retractor muscles extend into the foot from the visceral mass, the dorsal margin of the mantle cavity was limited ventrally by their presence, and its floor curved down to encompass them. In addition, these arcuate grooves may record the presence of left and right ctenidia, each having their efferent membranes attached ventrally to the mantle at its junction with the viscera, as in living Vetigastropods such as Pleurotomaria and Scissurella. If so, this implies that development of the ctenidium on the apical (right) side of the animal was reduced relative to that on the umbilical (left) side.
These observations suggest that Pelagiella had an extensible foot, differentiated from the visceral mass as in most multispiral crown group Gastropods. This feature may already have been present, prior to both torsion and spiral coiling of the shell, among the Helcionelloids. Whether or not in Pelagiella the foot terminated anteriorly in a well-developed head, with cephalic tentacles and other sensory organs such as those especially characteristic of Vetigastropods, is so far unknown.
Two remarkable specimens of Costipelagiella sp. have parts of a phosphatised digestive gland preserved within the last whorl of the shell. In most living Gastropods, the left lobe of the digestive gland is generally larger than that on the right. However, among living Vetigastropods such as Haliotis and the Scissurellids (Slit Snails), the digestive gland occurs on the right-hand side of the Animal. The disposition of the digestive gland observed in Costipelagiella sp. is thus consistent with space made available by reduction in size of the right ctenidium in these taxa and our reoriented reconstruction of the anatomy of Pelagiella. However, in the figures and reconstruction of these specimens the gland appears to occupy much of the last whorl of the shell, more space than would be expected even if it constitutes both the midgut and foregut portions of the gland.
The paired clusters of chaetae of Pelagiella exigua need to have been deployed anterolaterally, not posteriorly, to have functioned effectively in any likely role. Hence, the disposition of the chaetal appendages establishes the Animal’s anterior–posterior polarity in relation to its shell. This inference implies that Pelagiella’s shell coiling was endogastric as in Gastropods.
Model reconstructions of Pelagiella expressing torsion, with its chaetal arrays extended. (A) Right anterior lateral view, showing the apical surface of the shell and the anterior lobe of the foot (orange). (B) Posterior dorsal view, with the shell’s auricle (modestly developed here) on the left and diagrammatic representation of the gut (purple) running from the mouth (m) to the anus (a). (C) Left lateral, abapical view, showing the muscle mass of the foot extending upward to one of two pedal retractors and the gut running from the mouth to the anus, above and to the right of it. Whether the broadly hemiconical arrays of chaetae were extended convex-down or convex-up in life is uncertain. Bruce Runnager in Thomas et al. (2020).
In 1981 Bruce Runnager thought that the mantle cavity of Pelagiella atlantoides extended along the whole length of the foot, as in Chitons and Monoplacophorans, rather than being confined to the anterior region of the body as in Gastropods. Furthermore, he took asymmetric dextral coiling and its anatomical consequences to indicate that Pelagiella was an exogastric Helcionelloid, exhibiting about 10° of partial torsion. So, he reconstructed it as a transitional form with a limited developmental twist: counterclockwise as in Gastropod torsion, a mantle cavity extending along the entire right-hand side of the body, and paired posterior ctenidia. He suggested that the helically coiled shell evolved to lower the Animal’s centre of gravity and that partial torsion was required to balance the shell at an angle over the body axis, in accord with the ‘third law’ of Gastropod shell form. However, the adaptive function invoked to have required partial torsion cannot be sustained, as the stabilizing role of gravitational force is insignificant at the scale of a 2 mm organism living in seawater. The 'laws' of Gastropod shell form were originally intended to be applied only to marine Prosobranchs greater than 10 mm in length, due to the role of surface tension at smaller scales. Likewise, forces exerted by muscles and adhesion or friction retarding locomotion over a layer of mucus are large relative to that of gravity at this scale.
A reconstruction of Pelagiella as an Archaeobranchian Gastropod assumed the animal to have undergone full torsion in its development. This reconstruction is based primarily on supposed directions of flow of respiratory currents through the anterior mantle cavity, a single inhalant current entering the mantle cavity in the region of the auricle observed in some species on the umbilical side of the shell. The right ctenidium is shown reduced in size relative to that on the left. A large mass of gonad is associated with the small digestive gland shown on the right side, but not on the left. The latter features of this reconstruction are not based on observational evidence of fossils; they were inferred explicitly from the anatomies of living Gastropods, based upon the assumption that the Helcionelloids as Archaeobranchians, were in fact Gastropods.
Thomas et al.'s inference that the chaeta-bearing appendages of Pelagiella were necessarily anteroventral leads to a reconstruction such that the shell was endogastric, with its rapidly expanding aperture growing forward away from its apex. Asymmetry of the gills is based on the curved grooves on the interior of the shell of Pelagiella atlantoides. This asymmetry is inferred to have arisen from the combined effects of torsion and reallocation of space in the mantle cavity due to dextral coiling of the shell. It is consistent with the hypothesis that Pelagiella was a Gastropod, with an extended left gill comparable to those of Littorina and the Trochid Monodonta, and a reduced right gill as in many living Vetigastropods.
Thomas et al.'s reconstruction of Pelagiella as a Gastropod that underwent torsion, leading to asymmetric development of its ctenidia, is consistent in these respects with that last reconstruction. However, Thomas et al. regard the curvilinear grooves tracing attachment of the gills as evidence that was previously lacking. In their reconstruction, the main inhalent respiratory current is thought to have entered the mantle cavity along the left-lateral margin of the aperture, at a sinus (even a prominent auricle in some species) that gives the aperture of Pelagiella its unusual shape. This current passed either through the cluster of chaetae or adjacent to the auricle, where a sinus has sometimes been recognized. Likewise, another respiratory current is inferred to have entered the mantle cavity through or adjacent to the cluster of chaetae on the right. Circulation of these respiratory currents passed up through the gills and forward, above and between them on both sides of the viscera, passing the anus to exit right of the head.
The evidence now available (shell and body orientation, chaetae and the disposition of soft parts) constitutes the basis for Thomas et al.'s interpretation of Pelagiella as a Gastropod, with a dextrally coiled, anteriorly expanding shell, that underwent full torsion during its development. This is consistent with prior proposals that Aldanella, which appeared stratigraphically earlier, and the Pelagiellids may have been torted gastropods. However, this argument does not apply to Helcionelloids in general. These lack evidence of derived characters associated with torsion. Thomas et al. interpret the Helcionelloids as paraphyletic stem group Conchiferans, not crown group Gastropods.
Inferred evolution of Pelagiella in relation to its presumed emergence from the Helcionelloids. This scenario posits two alternative hypotheses: (1) chaetae were retained by at least some Helcionelloids and passed directly on to Pelagiella, potentially also to macluritaceans; in this case, they were lost independently in Tergomya, Cephalopoda, Gastropoda, and in Scaphopoda plus Bivalvia; (2) chaeta were lost among the earliest Conchiferans and later reactivated (atavisticly) before or after the branch giving rise to Pelagiella and possibly also the Macluritaceans. Thomas et al. (2020).
Newly elucidated, Pelagiella remains an enigma. On the one hand, Thomas et al. infer that it expresses torsion, the canonical derived character of crown group Gastropods. On the other, it bears chaetae, a plesiomorphic Lophotrochozoan character linking Molluscs to their common ancestry with Annelids and Brachiopods. Evidence from the fossil record provides an affirmative test of this hypothesis, which until now has been based on phylogenetic analyses of molecular and morphological data drawn from living organisms. Moreover, it extends its embrace to include the Gastropods, amongst which chaetae have not so far been recognized in any living or extinct taxon.
If the occurrence of chaetae in Pelagiella represents retention of a plesiomorphic character, transmitted by direct descent from an ancestral homologue, chaetae must have been retained by at least some Helcionelloids. A gradational series of shell forms can be recognized from Helcionelloids such as Latouchella, through Archaeospira, to Pelagiella. Hence chaetae could have been passed directly on to Pelagiella and perhaps even to the sedentary Ordovician Macluritaceans, whose means of feeding are as yet unknown. But there is at present no evidence of intermediary chaeta-bearing Helcionelloids or taxa linking the Pelagielloids to any later descendants.
If chaetae are plesiomorphic for Lophotrochozoans, this implies the retention of chaetae in at least the line leading ultimately to the Gastropoda and their loss in all other Conchiferan clades, as well as in lineages leading to the Sipuncula and Phoronida. Alternatively, chaetae may have reemerged in the Pelagiellida, having been lost in the stem of Conchifera. Living Cephalopods generally lack chaetae. But in juvenile Octopods, tufts of bristles known as Kölliker’s organs are remarkably similar to Annelid chaetae, both in form and in their development from basal chaetoblasts with microvillae. It has been suggested that Kölliker’s organs may be plesiomorphic in origin, hence exemplifying atavism, although this case has been attributed to convergence. The same inferences potentially apply to the re-emergence of chaetae in Pelagiella. In each case, a lost feature with a complex pattern of development reappears in a later clade after supposedly irrevocable loss. Neither hypothesis is evidently more parsimonious than the other. One involves multiple losses of a functionally significant character state; the other calls for reintroduction of a complex pattern. While direct descent can be corroborated by future discovery of intermediary taxa bearing chaetae, neither hypothesis can at this time be falsified. In fact, the difference between genomic atavism, achieved by reactivation of an inherited but suppressed developmental process, and evolutionary convergence achieved by deployment of the same developmental processes in a new context may be moot. They are effectively indistinguishable here.
By far more remarkable than the reappearance of chaetae in larval Octopods is the fact that spicules of a present- day larval Solenogaster share relatively complex patterns of serial and transverse organisation with those of sclerites of Halkieria and Wiwaxia. It has been proposed that these similarities arose from a shared, genetically based process of skeletogenesis, although the patterns have not so far been identified as being homologous. This also co-occurs in a Silurian Mollusc, the vermiform Multiplacophoran Acaenoplax, which shows features typical of Molluscs and Annelids. In the context of these observations, the hypothesis that the chaetae of Pelagiella arose as an atavistic revival of a pattern forming process that was suppressed in other Conchiferan lineages does not seem implausible.
In Lophotrochozoan taxa where chaetae and comparable features thought to be homologous with them occur, they develop in different anatomical and functional contexts. It is hard to see the evolution of these chitinous components in distantly related clades as outcomes of classical homology, as records of direct evolutionary continuity of form, in situ. Rather, they presumably represent the repeated emergence of chitinous novelties in different anatomical circumstances, employing homologous patterns of molecular development regulated by co-opted Hox and other genes, as now documented in Brachiopods and Annelids. In some cases, as apparently in Pelagiella, Annelids and Brachiopods, this deep homology involves more complete replication of a common molecular ‘toolkit’ than others.
If Pelagiella underwent torsion in its development, the emergence in the early Cambrian of this most fundamental of Gastropod characters supports the hypothesis that some of the earliest Molluscs were already Gastropods, as has been inferred from the shapes of their shells. The widely accepted notion that torsion and specialized modifications of the shell’s aperture which serve to improve adult sanitation evolved simultaneously during the latest Cambrian rests on the assumption that the latter adaptations were essential to accommodate torsion. Rather, it is more likely that torsion was acquired first, as a key adaptation that enabled a stem Gastropod to inhabit a tightly coiled spiral shell. Thomas et al. suggest that the earliest Gastropods did not diversify rapidly at first on account of collateral limitations arising from torsion. Later, in association with evolution to larger body sizes in the early Ordovician, Gastropods with shells bearing sinuses and slits evolved, resolving these circulatory difficulties. At that time they diversified rapidly, establishing the extant clades and thereby also the Gastropod crown group.
Whether or not Pelagiella and its near relatives constitute an immediate sister group of crown group Gastropods remains to be determined. Given a shell form some previous researchers have found hard to accommodate in the Gastropoda, and now the discovery that it had a pair of chaeta-bearing appendages, Pelagiella is more derived in its character states than Aldanella, especially if the chaetae of Pelagiella were atavistic and unique to its family. Hence, it appears that Aldanella, stratigraphically earlier in its first occurrence and closer to typical Gastropods in its shell form, is a more basal member of the clade and probably closer to the root stock from which crown group Gastropods ultimately evolved.
Gene regulatory mechanisms characteristic of Bilaterians are inferred to have emerged long before they were widely exploited in the divergence of crown group Metazoan phyla. The evolution of thermoregulatory or behaviourally advantageous feathers among Theropod Dinosaurs was an early precursor to the later emergence of winged birds, the prior adaptation being repurposed to serve a new function. Likewise, among the earliest Conchiferans, it now appears that torsion emerged as a key developmental innovation that only considerably later facilitated the evolutionary radiation of crown group Gastropods.
The unusual form of the shell of Pelagiella sets it apart from most Gastropods. This arises from peripheral expansion of its large and angular aperture, which is often variable in shape. It may be explained by the discovery that this small animal was equipped with two clusters of proportionately large chaetae, comparable in form and stiffness to capillary chaetae of Annelids, and up to half or more as long as the shell. These clusters of chaetae could apparently be extended as a pair of broad, forward and laterally directed, hemiconical arrays that were probably employed in feeding, either on Bacteria-rich mat-grounds or on the surfaces of Algal thalli. Chitinous chaetae are recognized for the first time as such among Conchiferans by Thomas et al. This evidence from the fossil record is consistent with genomic data that group Molluscs with Annelids and Brachiopods in the Lophotrochozoa. It also supports the hypothesis that chitinous chaetae constitute a deep homology of this clade.
The disposition of its chaeta-bearing appendages prompts a new reconstruction of the anatomy of Pelagiella. A long left ctenidium extended far back in the mantle cavity and a much shorter one was present on the right. This anatomical asymmetry, comparable to that of well-known living Vetigastropods, implies that Pelagiella underwent torsion during the course of its development. On this basis, Thomas et al. recognize Pelagiella as the earliest confirmed Stem Gastropod.
See also...
Follow Sciency Thoughts on Facebook.