Fungi are a Eukaryotic Kingdom that performs critical roles in the soil ecosystem. By forming vast microscopic filamentous networks (mycelium) in symbiosis with the roots of most Plants (mycorrhiza), Fungi can enhance rock weathering and help the nutrient supply of Plants, particularly in young, poorly evolved soils. Because of these abilities, ancestral Fungi were crucial partners of the first phototrophs that colonized land surfaces. Although these associations are acknowledged as a requirement of terrestrial invasion, the timing of this evolutionary transition is largely unknown. The Rhynie Cherts (407 million years old) with their superbly preserved Fungi are considered a milestone of Fungal fossil record and early colonisation of land. However, despite their Devonian age, Fungal remains in the Rhynie chert display a remarkable diversity, including members of Chytridiomycota, Blastocladiomycota, Glomeromycota, Mucoromycotina, and Ascomycota. Accordingly, molecular clock studies have placed the divergence of the main groups of Fungi within the Meso-Neoproterozoic. The terrestrialization of Fungi dates from sometime between the Ordovician (443 to 485 million years ago) and about 800 million years ago, while the earliest obligate biotrophic Glomeromycota fossils date from 455 to 460 million years ago. The large uncertainty in the timing of Fungal evolution and their transition to land essentially stems from the scarcity and the ambiguous nature of the Precambrian Fungi that are notoriously difficult to distinguish from Prokaryotic remains. A number of Precambrian remains (e.g. Caudosphaera, Hurionospora, Shuiyousphaeridium, Horodyskia, and Tappania), Lichen-like fossils from about 600 million years ago, as well as filament fragments and spores from 0.9 billion to 1 billion years ago in Arctic Canada, mycelium-like structures found in the Lakhanda Group of southeastern Siberia (1015 to 1025-million-years-old), or even an older structure found in 2.4 billion–year–old basalt from the Palaeoproterozoic Ongeluk Formation in South Africa were inferred to be of Fungal nature, yet their conclusive attribution to Fungi remains problematic.
In a paper published in the journal Science Advances on 22 January 2020, Steeve Bonneville of the Groupe de Biogéochimie et Modélisation du Système Terre at the Université Libre de Bruxelles, Franck Delpomdor of the Illinois State Geological Survey at the University of Illinois at Urbana-Champaign, Alain Préat, also of the Groupe de Biogéochimie et Modélisation du Système Terre at the Université Libre de Bruxelles, Clément Chevalier of the Center for Microscopy and Molecular Imaging at the Université Libre de Bruxelles, Tohru Araki and Majid Kazemian of the Diamond Light Source at the Harwell Science and Innovation Campus, Andrew Steele of the Geophysical Laboratory at the Carnegie Institution of Washington, Anja Schreiber and Richard Wirth of the German Research Centre for Geosciences, and Liane Benning, also of the German Research Centre for Geosciences, and of the Department of Earth Sciences at the Free University of Berlin, report the discovery of Fungi fossils in a 810 to 715-million-year-old (Tonian) dolomitic shale from the Mbuji-Mayi Supergroup of the Democratic Republic of Congo.
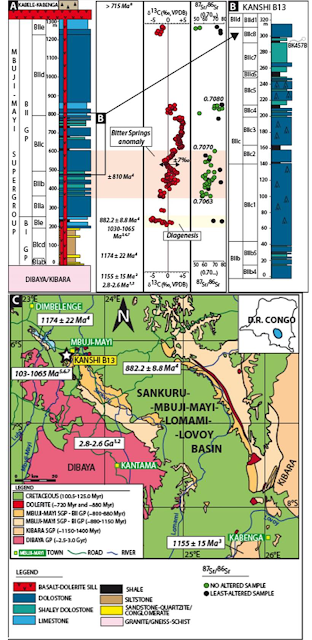
Bonneville et al. report the presence of filamentous networks attributed to Fungi in dolomitic shale rock of the Mbuji-Mayi Supergroup of the in the Sankuru-Mbuji-Mayi-Lomami-Lovoy Basin in the south-central part of the Democratic Republic of Congo. The filamentous fossils were identified in a thin section cut in BIIc8 level from a depth of 118.2 m in the Kanshi B13 drillcore (sample BK457b, core 118/4). The fungal networks were found embedded in a homogenous mineral matrix preserved as cylindric filaments. This three dimensional preservation results from an early dolomitic cementation, possibly due to schizohaline conditions (fluctuating hypersaline to fresh-water conditions), which hindered substantial compaction during burial. This BIIc8 dolomitic shale is a member of the BII group of the Mbuji-Mayi Supergroup that dominantly consists of shaley and Stromatolitic dolostones with dolomitic shale interbeds. The lower BII group (i.e., BIIa-BIIb) accumulated on a marine outer-to-inner ramp, while the palaeoenvironment of the upper BII (BIIc-BIId) evolved periodically toward a coastal, lagoon, perennial lacustrine pond with the deposition of a well-preserved fossil assemblage and the development of cyanobacterial mats and stratiform stromatolites. The transition to terrestrial palaeoenvironments in the upper BIIc is further evidenced by (i) the presence of coated pisoids and vadose/meteoric cements related to subaerial exposures, and (ii) a depleted, uniform light rare earth elements concentrations reflecting riverine water and continental sediment inputs. The Mbuji-Mayi Supergroup deposition time frame is constrained with an argon-argon age of 882.2 million years on a dolerite sill at the BI/BII group contact Within BIIc, the ratio of carbon¹³ to carbon¹² in carbonate rocks presents a large positive-to-negative excursion (about 7 parts per thousand) coeval to the Bitter Springs carbon¹³ anomaly at about 810 million years ago, while the age of the overlaying Kabele-Kabenga conglomerate is estimated at 715-million-years-old. Therefore, the estimated age of the fossiliferous dolomitic shales from BIIc8 is between about 810 and 715 million years.
Bonneville et al. document dark, nontranslucide, cylindrical filaments typically between 3.5 and 11.5 μm in width, extending over several hundreds of micrometers in length. These filaments sometimes evolve into dense interconnected networks of about 500 μm in diameter. In these mycelium-like structures, filaments exhibit multiple-order, high-angle branching and, possibly, anastomosing filaments, common features of Fungal networks yet rare for Prokaryotes. The width of filaments for extant and fossil Fungi can easily range from 2 to over 20 μm. Thus, the size of the fossil filaments observed here fits well with Fungal dimensions. However, size alone cannot be a reliable criterion to distinguish Fungal from Bacterial remains as Prokaryotes also form filaments with large dimensions. A previous study of rocks from the BIe-BIIc6 interval reported putative diversified Prokaryotic and Eukaryotic assemblages typical for the Tonian Period (1000 to 720 million years ago) with abundant Sphaeromorph and Acanthomorph Acritarchs. In the BIIc8 unit, three putative Fungal species, Eomycetopsis septata, Eomycetopsis cylindrica, and Eomycetopsis rugosa, were documented (depth interval between 118.4 and 122.90 m, i.e., 20 cm above Bonneville et al.'s fossiliferous rocks at 118.2 m). They were described as cylindrical filaments aggregated in groups and parenchyma-like masses with septate filaments. At a later stage, those fossils first attributed to Fungi were reassigned to tubular sheaths of Cyanobacteria. Thus, to unequivocally determine whether the filamentous networks that Bonneville et al. describe represent Neoproterozoic Fungal or Cyanobacterial remains, an in-depth chemical characterisation was required.
One of the major constituents of fungal cell wall is chitin, a biopolymer of β 1–4–bonded N-acetylglucosamine, which is abundant in Fungi and more generally in Eukaryote taxa (i.e., Ciliates, Arthropods, Chrysophytes, and Diatoms), yet absent in Prokaryotic organisms such as Actinobacteria (which can also form mycelium-like structures). Hence, chitin alone cannot be used as a unique defining trait of Fungi; however, the chitin-producing organisms listed above are morphologically distinct from our fossils in that they do not form mycelial networks, and the presence of chitin within our filaments would be a strong argument for a fungal affinity. Chitin can be preserved throughout geological time. It has been detected in Insects in an Oligocene shale and in Fungi preserved in Cretaceous amber, and it was even found in a 505-million-year-old marine Sponge from the Burgess Shale. Those discoveries support the idea that the main control of chitin preservation is not age but rather the environment, with organic matter–rich sediment being ideal for preservation.
Bonneville et al. document the presence of chitin in the fossil filaments by staining with wheat germ agglutinin conjugated with fluorescein isothyocyanate, a highly specific dye of N-acetylglucosamine trimers. Wheat germ agglutinin conjugated with fluorescein isothyocyanate has been widely used to discriminate between Fungal hyphae and filamentous Prokaryotes in soils, sediments, and rocks, as well as in Cyanobacteria-Fungus endosymbiosis (i.e., Geosiphon-Nostoc) and has also been applied as a Fungal marker in Eocene basalt and in Cretaceous amber. Bonneville et al. used confocal laser scanning fluorescence microscopy and observed that the wheat germ agglutinin conjugated with fluorescein isothyocyanate binds to extensive portions of the mycelial structure. In the mycelium, wheat germ agglutinin conjugated with fluorescein isothyocyanate was detected in the cell wall of filaments that appear cylindrical, suggesting a preservation in three dimensions of the remains. A few septa were stained as well and, on some instances, what appear to be 'incomplete septa' (pseudosepta) that do not fully separate adjacent cells. Confocal views also allow observing some putative anastomosis of fungal filaments. In contrast to Bonneville et al.'s observations in filamentous networks, staining was negative when wheat germ agglutinin conjugated with fluorescein isothyocyanate was exposed to inclusions of 'nonfungal; organic matter in BIIc6 shale, indicating that wheat germ agglutinin conjugated with fluorescein isothyocyanate maintained its high-specificity binding when exposed to ancient, mature organic matter. Considering the Fungus-like morphology, the colocalisation/binding of the wheat germ agglutinin conjugated with fluorescein isothyocyanate with/to cell wall/septa and its specific affinity for chitin, Bonneville et al. assert that the staining can be used as a clear indicator of chitinous remnants. Simultaneously, the detection of vestigial chitin in these filamentous fossils is a strong indication of their Fungal origin.
Bonneville et al. report the presence of ancient Fungal filaments and mycelium-like structures preserved in a Neoproterozoic dolomitic shale (between. 810 and 715-million-years-old). This discovery proves definitively that filaments, found earlier, which were later reassessed as Cyanobacterial tubular sheaths, were in fact remains of fungal origin. Hence, this finding extends the record of Fungi fossils by more than 250 Ma with respect to previously reported oldest Fungi fossil dating from the mid-Ordovician (460 to 55-million-years-old).
In Bonneville et al.'s view, the observed septation of the hypha does not indicate a Dikaryan affinity, which appears unlikely considering the Neoproterozoic age of the remains and the much later timing of the Dikarya evolution (Basidiomycota and Ascomycota). Instead, the low frequency of the septation and also the presence of incomplete septa (pseudosepta) in the fossil filaments resemble more Blastocladiomycota hypha. This basal phylum contains both parasitic and saprotrophic Fungal species and is found in both aquatic and terrestrial environments. Hence, considering the very shallow, periodically subaerially exposed, lacustrine nature of the upper BIIc palaeoenvironment where the Fungal remains were discovered, Bonneville et al hypothesise that those Fungi were in association with (either parasitic or symbiotic) or decomposers of an early photosynthetic community in transition toward terrestrial life. Ephemeral ponds, with their frequent wet-drying cycles (e.g., along the shores), might have been favorable environments for physical interactions between Fungi and Algae. Those early associations with photosynthetic organisms were presumably restricted to free living, and possibly Lichenized, Green Algae or Cyanobacteria, which may have represented a primitive form of cryptogamic cover. Although no such symbiotic relationships have been observed directly by Bonneville et al., it is worth noting that the upper BIIc shale beds, in addition to Fungal remains, exhibit an abundant and diverse assemblage of microfossils including 11 Eukaryotic, Acantomorph, presumably Algal species. Hence, Bonneville et al.'s study represents the oldest, documented Fungi to date and pushes well into the Neoproterozoic the possibility that Fungi helped to colonise land surface, almost 300 million years before the first evidence of Land Plants. Overall, Bonneville et al.'s discoveries also lend support to previous assumptions regarding the role of Fungi in land colonisation and, by extension, on the evolution of Earth’s biogeochemical cycles. Neoproterozoic soil development driven by early terrestrial biota has generated increasing amounts of
pedogenic clay minerals that helped to stabilize organic matter and enhanced carbon burial, which, in turn, contributed to the stepwise oxygenation of the Late Precambrian.
See also...
Geological map of the Sankuru-Mbuji-Mayi-Lomami-Lovoy Basin and synthetic stratigraphic and chemostratigraphic column of the Mbuji-Mayi Supergroup. (A) Synthetic stratigraphic column of the Mbuji-Mayi Supergroup in the Democratic Republic of Congo combined with composite chemostratigraphic-isotope profiles. Note the presence of the carbon isotope anomaly (±7‰) in the carbonate rocks in the lower BIIc subgroup coeval to the Bitter Spring anomaly. On top of the BIIc subgroup, the Kabele-Kabenga conglomerate is stratigraphically-equivalent to the Grand Conglomérat in Katanga (over 715-million-years-old). Bonneville et al. (2020).
Bonneville et al. document dark, nontranslucide, cylindrical filaments typically between 3.5 and 11.5 μm in width, extending over several hundreds of micrometers in length. These filaments sometimes evolve into dense interconnected networks of about 500 μm in diameter. In these mycelium-like structures, filaments exhibit multiple-order, high-angle branching and, possibly, anastomosing filaments, common features of Fungal networks yet rare for Prokaryotes. The width of filaments for extant and fossil Fungi can easily range from 2 to over 20 μm. Thus, the size of the fossil filaments observed here fits well with Fungal dimensions. However, size alone cannot be a reliable criterion to distinguish Fungal from Bacterial remains as Prokaryotes also form filaments with large dimensions. A previous study of rocks from the BIe-BIIc6 interval reported putative diversified Prokaryotic and Eukaryotic assemblages typical for the Tonian Period (1000 to 720 million years ago) with abundant Sphaeromorph and Acanthomorph Acritarchs. In the BIIc8 unit, three putative Fungal species, Eomycetopsis septata, Eomycetopsis cylindrica, and Eomycetopsis rugosa, were documented (depth interval between 118.4 and 122.90 m, i.e., 20 cm above Bonneville et al.'s fossiliferous rocks at 118.2 m). They were described as cylindrical filaments aggregated in groups and parenchyma-like masses with septate filaments. At a later stage, those fossils first attributed to Fungi were reassigned to tubular sheaths of Cyanobacteria. Thus, to unequivocally determine whether the filamentous networks that Bonneville et al. describe represent Neoproterozoic Fungal or Cyanobacterial remains, an in-depth chemical characterisation was required.
Textures and structures observed via
light or scanning electron microscopy of a thin section from the
fossiliferous dolomitic shale rock from layer BIIc8 of the Mbuji-Mayi
Supergroup. (A) Composite image of light microscopy views of dark,
nontranslucide, interconnected filaments forming part of a large
mycelium-like structure covering about 0.2 mm². (C) Scanning electron
microscopy micrograph illustrating the presence of cells (roughly 20 to
25 μm in length) regularly spaced by putative septa or pseudosepta
(white arrows) and showing putative anastomoses (red arrow). (D) Light microscopy
image of a mycelium network of dark-colored filaments with Y- and
T-branching (red arrows); black arrow marks the position of an focused
ion beam foil #4984 cut across a filament in the thin section. Inset
shows a portion of mycelial network composed of dark, nontranslucide
filaments branching at a right angle. (E) Histogram showing the width
frequency distribution of filaments, with the solid line depicting the
median at 5.5 μm (the dashed line as first and third quartiles at 4.5 and 6.7 μm). (F) Encrusted aspect of these fungal networks and the absence of tunneling patterns suggesting the syngenicity of these remains. Bonneville et al. (2020).
median at 5.5 μm (the dashed line as first and third quartiles at 4.5 and 6.7 μm). (F) Encrusted aspect of these fungal networks and the absence of tunneling patterns suggesting the syngenicity of these remains. Bonneville et al. (2020).
One of the major constituents of fungal cell wall is chitin, a biopolymer of β 1–4–bonded N-acetylglucosamine, which is abundant in Fungi and more generally in Eukaryote taxa (i.e., Ciliates, Arthropods, Chrysophytes, and Diatoms), yet absent in Prokaryotic organisms such as Actinobacteria (which can also form mycelium-like structures). Hence, chitin alone cannot be used as a unique defining trait of Fungi; however, the chitin-producing organisms listed above are morphologically distinct from our fossils in that they do not form mycelial networks, and the presence of chitin within our filaments would be a strong argument for a fungal affinity. Chitin can be preserved throughout geological time. It has been detected in Insects in an Oligocene shale and in Fungi preserved in Cretaceous amber, and it was even found in a 505-million-year-old marine Sponge from the Burgess Shale. Those discoveries support the idea that the main control of chitin preservation is not age but rather the environment, with organic matter–rich sediment being ideal for preservation.
High-resolution scanning electron microscope micrographs of the mycelial networks. (A)-(C) High-resolution scanning electron microscopemicrographs
of the mycelial network with detailed views of anastomosing filaments
(a arrows), ‘Y’ (b arrow) and high angle ‘T’ (c arrows) (D) Stitched
light microscope micrographs showing the density of branching in the
Fungal network. Bonneville et al. (2020).
Bonneville et al. document the presence of chitin in the fossil filaments by staining with wheat germ agglutinin conjugated with fluorescein isothyocyanate, a highly specific dye of N-acetylglucosamine trimers. Wheat germ agglutinin conjugated with fluorescein isothyocyanate has been widely used to discriminate between Fungal hyphae and filamentous Prokaryotes in soils, sediments, and rocks, as well as in Cyanobacteria-Fungus endosymbiosis (i.e., Geosiphon-Nostoc) and has also been applied as a Fungal marker in Eocene basalt and in Cretaceous amber. Bonneville et al. used confocal laser scanning fluorescence microscopy and observed that the wheat germ agglutinin conjugated with fluorescein isothyocyanate binds to extensive portions of the mycelial structure. In the mycelium, wheat germ agglutinin conjugated with fluorescein isothyocyanate was detected in the cell wall of filaments that appear cylindrical, suggesting a preservation in three dimensions of the remains. A few septa were stained as well and, on some instances, what appear to be 'incomplete septa' (pseudosepta) that do not fully separate adjacent cells. Confocal views also allow observing some putative anastomosis of fungal filaments. In contrast to Bonneville et al.'s observations in filamentous networks, staining was negative when wheat germ agglutinin conjugated with fluorescein isothyocyanate was exposed to inclusions of 'nonfungal; organic matter in BIIc6 shale, indicating that wheat germ agglutinin conjugated with fluorescein isothyocyanate maintained its high-specificity binding when exposed to ancient, mature organic matter. Considering the Fungus-like morphology, the colocalisation/binding of the wheat germ agglutinin conjugated with fluorescein isothyocyanate with/to cell wall/septa and its specific affinity for chitin, Bonneville et al. assert that the staining can be used as a clear indicator of chitinous remnants. Simultaneously, the detection of vestigial chitin in these filamentous fossils is a strong indication of their Fungal origin.
Confocal laser scanning fluorescence microscopy using wheat germ
agglutinin conjugated with fluorescein isothyocyanate of mycelium-like
structure illustrated above. Overview image in (A) shows the absence of
large natural autofluorescence (i.e., without wheat germ agglutinin
conjugated with fluorescein isothyocyanate) of the thin section. In
contrast, with wheat germ agglutinin conjugated with fluorescein
isothyocyanate labeling, the same area exhibits filamentous,
mycelium-like structure visible in (B) (top section). (C) to (F)
High-resolution confocal fluorescence views of the mycelium-like
structures. The wheat germ agglutinin conjugated with fluorescein
isothyocyanate binds specifically on the cell wall of the fossil
filaments, which appear cylindrical (as evidenced by a rounded cross
section of filaments). Several septa (s) are also stained. Note in (C)
that the arrows highlight septa present in putative anastomosing
filaments (a) (arrows; images are mirrored as confocal microscope is
inverted). Insets in (F) illustrate septa (ps) with perforation or
'bulged', which resembles pseudosepta. Scale bars are 10 μm (C) to (F).
Bonneville et al. (2020).
Bonneville et al. report the presence of ancient Fungal filaments and mycelium-like structures preserved in a Neoproterozoic dolomitic shale (between. 810 and 715-million-years-old). This discovery proves definitively that filaments, found earlier, which were later reassessed as Cyanobacterial tubular sheaths, were in fact remains of fungal origin. Hence, this finding extends the record of Fungi fossils by more than 250 Ma with respect to previously reported oldest Fungi fossil dating from the mid-Ordovician (460 to 55-million-years-old).
In Bonneville et al.'s view, the observed septation of the hypha does not indicate a Dikaryan affinity, which appears unlikely considering the Neoproterozoic age of the remains and the much later timing of the Dikarya evolution (Basidiomycota and Ascomycota). Instead, the low frequency of the septation and also the presence of incomplete septa (pseudosepta) in the fossil filaments resemble more Blastocladiomycota hypha. This basal phylum contains both parasitic and saprotrophic Fungal species and is found in both aquatic and terrestrial environments. Hence, considering the very shallow, periodically subaerially exposed, lacustrine nature of the upper BIIc palaeoenvironment where the Fungal remains were discovered, Bonneville et al hypothesise that those Fungi were in association with (either parasitic or symbiotic) or decomposers of an early photosynthetic community in transition toward terrestrial life. Ephemeral ponds, with their frequent wet-drying cycles (e.g., along the shores), might have been favorable environments for physical interactions between Fungi and Algae. Those early associations with photosynthetic organisms were presumably restricted to free living, and possibly Lichenized, Green Algae or Cyanobacteria, which may have represented a primitive form of cryptogamic cover. Although no such symbiotic relationships have been observed directly by Bonneville et al., it is worth noting that the upper BIIc shale beds, in addition to Fungal remains, exhibit an abundant and diverse assemblage of microfossils including 11 Eukaryotic, Acantomorph, presumably Algal species. Hence, Bonneville et al.'s study represents the oldest, documented Fungi to date and pushes well into the Neoproterozoic the possibility that Fungi helped to colonise land surface, almost 300 million years before the first evidence of Land Plants. Overall, Bonneville et al.'s discoveries also lend support to previous assumptions regarding the role of Fungi in land colonisation and, by extension, on the evolution of Earth’s biogeochemical cycles. Neoproterozoic soil development driven by early terrestrial biota has generated increasing amounts of
pedogenic clay minerals that helped to stabilize organic matter and enhanced carbon burial, which, in turn, contributed to the stepwise oxygenation of the Late Precambrian.
See also...
Follow Sciency Thoughts on Facebook.