Primeval Microbes likely required small organic molecules to act as building blocks for biomass and as catabolic substrates for heterotrophic metabolism. A potential source of such compounds includes recycled and redistributed organic matter from pre-existing biomass. In addition, ample exogenous organic matter probably had been delivered to the early Earth by interplanetary dust particles and meteorites. Experiments have also shown that organic molecules relevant for primordial life can be formed by synthesising organic compounds from inorganic atmospheric gases. As important, endogenous synthesis and processing of organic molecules could have occurred in marine and terrestrial (i.e. hot spring) hydrothermal environments. In such settings, organic molecules may form, or react, at elevated temperatures and pressures within the steady flow of inorganic hydrothermal chemistry (e.g. hydrogen sulphide, carbon dioxide, molecular hydrogen). One hypothesis on organic synthesis at hydrothermal sites suggests that the reaction of iron(II) sulphide to pyrite with hydrogen sulphide drives the reduction of carbon dioxide to organic molecules. Moreover, a primordial carbon fixation mechanism involving the reaction of carbon monoxide with methanethiol on catalytic metal (nickel or iron) sulphide surfaces could be demonstrated in the laboratory under hydrothermal conditions. This experiment produced an activated form of acetic acid that represents a plausible building block for further organic synthesis, for example, into acyl lipids. As yet, however, such distinctive organic molecules have not been found in rocks that directly testify to the emergence of life on our planet.
The roughly 3.5 billion-year-old Dresser Formation (Pilbara Craton, Western Australia) is one of the most important windows into hydrothermal habitats on early Earth. The rocks are only mildly metamorphosed (prehnite-pumpellyite to lower greenschist facies) and still preserve numerous putative biosignatures, including Stromatolites, microfossils, and isotopic anomalies. Further, cherts and barites of the Dresser Formation contain kerogenous organic material of supposedly biological origin. Detailed field mapping, petrographic observations, and mineralogical analyses revealed that the Dresser Formation was formed in a hydrothermal setting, most likely a volcanic caldera. Thus, it appears plausible that organisms in the Dresser environments grew chemotrophically, fuelled by hydrothermal fluids that delivered inorganic and organic substrates. Indeed, stable carbon and sulphur isotopic anomalies indicate methanogenic and sulphur-disproportionating Microbes as key players in these early Microbial communities, although the exact metabolisms still await further evidence and testing.
Cherts and barites of the Dresser Formation contain abundant primary fluid inclusions, that is, fluids and/or gases entrapped in minerals. These fluid inclusions represent a valuable archive, as their chemistry can potentially be preserved for billions of years. Barite appears to be a particularly robust host mineral because of its low solubility and high stability under a wide range of pressure, temperature and redox conditions. Therefore, fluid inclusions in the Dresser barites are excellent candidates in the search for organic molecules that once supported Microbial life. Previous work identified water, carbon dioxide, hydrogen sulphide, and minor methane as the main inorganic constituents of the fluid inclusions in Dresser barites. However, the content of organic molecules, potential key ingredients for early life, is as yet unknown.
In a paper published in the journal Nature Communications on 17 February 2021, Helge Mißbach of Geobiology at the University of Göttingen, and Geobiology at the University of Cologne, Jan-Peter Duda, also of Geobiology at the University of Göttingen, the 'Origin of Life' Group at the Göttingen Academy of Sciences and Humanities, and Sedimentology & Organic Geochemistry at the University of Tübingen, Alfons van den Kerkhof of Applied Geology at the University of Göttingen, Volker Lüders of the GFZ German Research Centre for Geosciences, Andreas Pack of the Isotope Geology Divison at the University of Göttingen, Joachim Reitner, also of Geobiology at the University of Göttingen, and the 'Origin of Life' Group at the Göttingen Academy of Sciences and Humanities, and Volker Thiel, once again of Geobiology at the University of Göttingen, report on the presence of biologically-relevant primordial organic molecules in primary fluid inclusions trapped in barites of the roughly 3.5 billion-year-old Dresser Formation. To explore the full range of volatiles, Mißbach et al. combined gas chromatography–mass spectrometry, microthermometry, fluid inclusion petrography, and stable isotope analysis. Their findings reveal an intriguing diversity of organic molecules with known or inferred metabolic relevance and provide a strong clue as to how ancient hydrothermal fluids sustained Microbial life about 3.5 billion years ago.
The Dresser Formation contains thick barite units with colours ranging from white and grey to black. Black barites exhibit coarse crystalline textures and yield a strong hydrogen sulphide odour when freshly crushed. The sedimentary black barite studied here was sampled in the Dresser mine, where it was interbedded with originally sulphidic Stromatolites. Field and petrographic evidence clearly suggest a primary origin of the barite (e.g. no progressive replacement of stromatolite interbeds, no relicts of potential precursor materials within the barite). Thin section analysis revealed the presence of abundant primary and rare secondary inclusions. Most primary fluid inclusions are small (about 10 μm), translucent, and often oriented parallel to planes of barite crystals, thereby tracing succeeding growth phases. Morphologies of some fluid inclusions indicated necking down, which is a typical modification under stress conditions after crystallisation. These inclusions are typically stretched and may split up in segments that then usually show different composition and density.

Study area and field evidence. Location of the Dresser mine in Western Australia near Marble Bar (a) and black barite associated with originally sulphidic Stromatolites at the sampling site (b) and in the working area (c). The close association between the inclusion-bearing black barites and Stromatolites suggests that hydrothermal fluids might have influenced ancient microbial communities. Mißbach et al. (2021).
The fluid inclusions were analysed optically on a heating-freezing stage and by Raman spectroscopy. The black barites contain aqueous carbonic-sulfuric and non-aqueous carbonic-sulfuric fluid inclusions (hereafter, aqueous, and non-aqueous, respectively). Aqueous inclusions show highly variable water volume fractions of 0.1–1. At room temperature, they typically exhibit a double meniscus, indicating the presence of three phases: water + another (carbon dioxide–hydrogen sulphide-rich) liquid + vapour. In some cases, the other liquid is only visible during cooling runs. In comparison, non-aqueous fluid inclusions usually contain a carbon dioxie–hydrogen-sulphide-rich liquid and a vapour phase, although the liquid phase is sometimes absent at room temperature.

Fluid inclusions in representative black barites from the Dresser mine. (a), (b) Thin section images (reflected light) showing primary fluid inclusion trails parallel to barite crystal growth bands (marked by black arrows). (c) Thin section image (transmitted light) showing primary fluid inclusions which are dispersed or oriented parallel to barite crystal growth bands (exemplified by dashed line). The image also shows a minor secondary inclusion trail (marked by black arrow). (d) Thick section image (transmitted light) of an aqueous carbonic-sulfuric fluid inclusion containing three volatile phases (including hydrogen sulphide), plus pyrite, native sulfur, and strontianite as solid phases. (e) Thick section image (transmitted light) of a non-aqueous fluid inclusion bearing a vapour phase, native sulphur, and kerogen. These fluid inclusions are usually rich in hydrogen sulphide. V vapour/gas, Lw liquid water, L other liquid (e.g. CO2carbon dioxide). Organic compounds and gases preserved in these primary fluid inclusions could have provided a substrate to primordial microbial life in the Dresser Formation. Mißbach et al. (2021).
Both types of fluid inclusions typically contain solid daughter phases. Aqueous inclusions usually contain strontianite and sulphur as daughter crystals. Varieties with pure carbon dioxide in the vapour phase (volume fractions of about 0.9) may additionally include anatase, pyrite, and possibly also halite. In non-aqueous inclusions, typical daughter phases are sulphur, kerogen and, in few cases, halite.
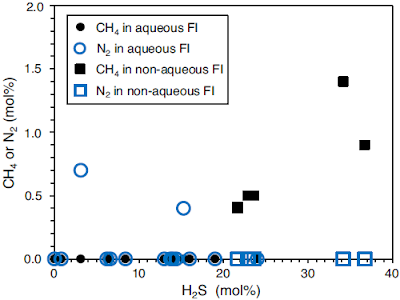
The main gas components in both fluid inclusion types are carbon dioxide and hydrogen sulphide, accompanied by minor amounts of methane, nitrogen, and carbonyl sulphide. Aqueous fluid inclusions contain less hydrogen sulphide than non-aqueous fluid inclusions (0–24% by molarity and 21–36% by molarity, respectively). Furthermore, aqueous fluid inclusions typically enclose up to 1% by molarity nitrogen, which is not present in non-aqueous fluid inclusions. Instead, non-aqueous fluid inclusions additionally contain small amounts of methane (less than 2% by molarity).
Gas compositions of fluid inclusions in black barites as measured by Raman analysis. 0 % hydrogen sulphide (H₂S) implies that the fluid inclusion largely contains carbon dioxide (CO₂). FI fluid inclusions. Mißbach et al. (2021).
Aqueous fluid inclusions typically reveal liquid compositions ranging from pure water to more saline solutions with 14% by weight sodium chloride-equivalents. Higher salinities of up to 25% by weight sodium chloride-equivalents are rare. The corresponding ice melting temperatures vary between 0°C and −26°C (peak at −7 °C). Aqueous fluid inclusions form clathrates upon freezing and subsequent melting between 7°C (pure carbon dioxide) and 20°C (hydrogen sulphide-rich). Total homogenisation temperatures, describing the minimum temperature of fluid entrapment, range from 100 to 195°C, with a maximum between 110 and 150°C. Most fluid inclusions decrepitate at temperatures of over 230°C.

Bar plots showing phase transition temperatures. From top to bottom: (i) homogenisation of the non-aqueous phase (total homogenisation temperature carbon dioxide-hydrogen sulphide), (ii) melting of carbon dioxide and hydrogen sulphide (melt temperature carbon dioxide-hydrogen sulphide), (iii) ice and clathrate melting (melt temperature ice/(melt temperature ice, metastable, and melt temperature clathrates, respectively), (iv) total homogenisation and decrepitation (total homogenisation and total decrepitation, respectively) temperatures. C, critical; V, vapour, L, liquid. Mißbach et al. (2021).
Non-aqueous fluid inclusions show total homogenisation temperatures (carbon dioxide-hydrogen sulphide) between 16 and 38°C. Those containing higher concentrations of hydrogen sulphide typically homogenise at the higher end of this range, that is, above the critical temperature of carbon dioxide (31.1°C). Phases usually homogenise to liquid, and only rarely to the gas or critical phase. During cooling runs, the subsequent melting of solid carbon dioxide and hydrogen sulphide can be observed at lower temperatures compared to the pure compounds (−56.6°C and −83.6°C, respectively).
Mißbach et al.'s data demonstrate that the majority of aqueous and nonaqueous inclusions formed during crystal growth (i.e. primary inclusions). Thus, fluids must have been immiscible at the time of encapsulation, and experienced identical trapping and homogenisation temperatures (i.e. heterogeneous trapping). Therefore, no pressure correction is necessary.
Online analyses of black barite fragments using thermal decrepitation-gas chromatography–mass spectrometry yielded high amounts of carbon dioxide, hydrogen sulphide, and water, thus confirming results from Raman analysis on fluid inclusions. The diversity and intensity of compounds was considerably higher in the 250°C than in the 150°C experiment. This finding is consistent with the microthermometry data revealing that most fluid inclusions remain intact up to about 230°C.

Total ion current chromatogram of volatile compounds from black barite fluid inclusions as detected by thermal decrepitation/desorption thermal decrepitation-gas chromatography–mass spectrometry analysis at 250 C. Inserts (a), (b) represent enlargements of respective areas in the chromatogram marked by dashed lines. Triangles denote oxygen-bearing compounds, circles denote aromatic hydrocarbons and stars denote sulfur-bearing compounds. n-Hexane (Hex) was used as a retention time standard (RT std.). COS carbonyl sulphide, Ea ethanal, MT methanethiol, Bu but-1-ene, Pa prop-2-enal, Pa’ propanal, ET ethanethiol, MSM (methylsulfanyl)methane, Po propan-2-one, Ba but-2-enal, Ox oxolane, TP thiophene, B benzene, Ac acetic acid, TL thiolane. Note the presence of methanethiol and acetic acid, the stable building blocks of activated acetic acid. Mißbach et al. (2021).
Offline analysis using solid phase micro extraction-gas chromatography–mass spectrometry revealed numerous organic molecules containing oxygen (aldehydes, ketones, acetic acid, oxolane) and/or sulphur (thiophene, thiols, organic polysulphanes), along with some aromatic hydrocarbons (e.g. benzene, alkylbenzenes). Compounds detected with both analytical techniques showed a lower abundance in olid phase micro extraction-gas chromatography–mass spectrometry as compared to thermal decrepitation-gas chromatography–mass spectrometry at 250°C. On the other hand, solid phase micro extraction-gas chromatography–mass spectrometry yielded a considerably greater diversity of compounds, especially in the higher molecular weight range. The absence of carbon doxide and hydrogen sulphide in the solid phase micro extraction-gas chromatography–mass spectrometry runs is due to an analytical bias, as these compounds do not adsorb onto the solid phase micro extraction fibre.

Total ion current chromatogram of volatile compounds from black barite fluid inclusions as obtained by solid phase micro extraction solid phase micro extraction-gas chromatography–mass spectrometry. Inserts (a)–(c) represent enlargements of respective areas in the chromatogram marked by dashed lines. Triangles denote oxygen-bearing compounds, circles denote aromatic hydrocarbons and stars denote sulphur-bearing compounds. n-Hexane (Hex) was used as a retention time standard (RT std.). COS carbonyl sulfide, Ea ethanal, MT methanethiol, Pa prop-2-enal, Pa’ propanal, ET ethanethiol, MSM (methylsulfanyl)methane, Po propan-2-one, Ba but-2-enal, Ox oxolane, Bo butan-2-one, TP thiophene, B benzene, Ac acetic acid, MB 3-methylbutan-2-one, Mxp 1-methoxypropan-2-ol, Pe pentanal, MDSM (methyldisulfanyl)methane, To toluene, MP 4-methylpentan-2-one, Ha hexanal, MEDS (methyldisulfanyl)ethane, Xy I p-xylene, Xy II m-xylene, Pac 1-methoxyprop-2-yl acetate, Xy III o-xylene, Sty styrene, CH cyclohexanone, Hp heptanal, BA benzaldehyde, MTSM (methyltrisulfanyl)methane, TMB I 1,3,5-trimethyl benzene, TMB II 1,2,4-trimethyl benzene, MH 6-methylheptan-3-one, TMB III 1,2,3-trimethyl benzene. Note the higher diversity of compounds as compared to thermal decrepitation/desorption analysis. Oxygen- and sulfur-bearing organic compounds may have provided substrates for microbial life in the Dresser Formation. Mißbach et al. (2021).
The mean total organic carbon content of the black barite is 0.31% by weight. Stable carbon isotope analysis revealed a mean proportional value of −27.6±0.6‰ carbon¹³ in total organic carbon, compared to the Vienna Pee Dee Belemnite standard. Offline analysis revealed porportions of carbon¹³ and oxygen¹⁶ in carbon dioxide values of −10.0±0.3‰ and 34.1±0.6‰, respectively, compared to the Vienna Pee Dee Belemnite and Vienna Standard Mean Ocean Water standards. Online analyses yielded porportions of carbon¹³ in carbon dioxide values ranging from −14.3 to −8.9±0.3 ‰ for black barites (mean = –10.3 ‰) and from −8.6 to −4.0±0.3‰ for grey barites (mean = −6.3 ‰). Thus, black barites are consistently more depleted in carbon¹³ than their grey counterparts. In all cases, methan and nitrogen contents were too low for stable isotope analyses (less than 2% by molarity).

Molecular structures of oxygen-bearing compounds, aromatic hydrocarbons, and sulphur-bearing compounds found in black barite fluid inclusions. Mißbach et al. (2021).
The Black barites studied by Mißbach et al. classify as primary hydrothermal sediments that precipitated from discharging fluids. This interpretation is additionally supported by the facts that (i) the originally sulphidic Stromatolite interbeds are still largely intact and show no indications for a progressive replacement by barite and (ii) that the barite does not contain relicts of potential precursor materials. Mißbach et al.'s observations are therefore consistent with earlier studies that argued for a primary, synsedimentary origin of the Dresser barites analysed herein (i.e. precipitation in surface environments linked to hydrothermal activity).

Distribution of stable carbon isotope signatures of carbon dioxide from black and grey barite fluid inclusions. Reproducibility of the stable isotope measurements is 0.3‰. A total of 11 black barite samples and 11 grey barite samples was analysed. The relatively low proportional carbon¹³ values in the black barites possibly reflect the addition of a biomass-derived carbon component to the fluids. Mißbach et al. (2021).
Barite is highly chemically stable under a wide range of geological conditions. Hence, barite-hosted fluid inclusions can preserve information on the original composition of hydrothermal fluids. The black and grey barites from the Dresser Formation primarily grew as coarse crystals and contain abundant primary fluid inclusions. Most fluid inclusions show no indication of post-entrapment modification. The results are reproducible and total homogenisation temperature values (100–195°C) are internally consistent for different coevolutionary fluid inclusions. The measured total homogenisation temperature is in line with (i) formation temperatures estimated for coexisting cherts (100–200°C), and (iii) maximum formation temperatures of barite-hosted fluid inclusions in a modern hydrothermal system (the Jade hydrothermal field in the Izena Hole, mid-Okinawa Trough, 150–200°C).
The aqueous and non-aqueous fluid inclusions distinguished herein appear to include those described in earlier studies. Particularly key-characteristics such as sizes (5–30 μm), ice and clathrate melting temperatures (−7.5 to −0.6°C and −0.9 to 19.2°C, respectively), and the fundamental volatile inventories (carbon dioxide, water, hydrogen sulphide, methane) are all remarkably similar. A notable exception is the presence of trace amounts of nitrogen in some of the aqueous fluid inclusions, which has not been reported previously.
The presence of aqueous and non-aqueous fluid inclusions can be explained by the presence of two coexisting fluids at the time of trapping as a result of phase separation from boiling fluids during cooling (effervescence). Hence, the major fluid composition of the black barites can be considered primary. However, there are indications that a few fluid inclusions were locally modified immediately after emplacement (e.g. necking down after crystallisation), explaining the wide variations observed in total homogenisation temperatures. This information is not relevant to the interpretation of the fluids as being primary, because they would be trapped again instantly with their overall composition remaining unchanged.
Organic molecules detected by gas chromatography–mass spectrometry are derived from the fluid inclusions as evidenced by (i) clean pre-analysis blanks, (ii) retrieval of products exclusively after grinding of barite, (iii) reproducibility of the results from five thermal decrepitation-gas chromatography–mass spectrometry and seven solid phase micro extraction-gas chromatography–mass spectrometry experiments, (iv) presence of highly volatile compounds in gas chromatography–mass spectrometry analyses, (v) consistency of data obtained by independent analytical techniques (Raman spectroscopy vs. gas chromatography–mass spectrometry), (vi) temperature dependency of product yields, meaning that higher temperature analyses above the decrepitation temperature of fluid inclusions result in higher abundances (thermal decrepitation-gas chromatography–mass spectrometry 150°C vs. thermal decrepitation-gas chromatography–mass spectrometry 250°C), and (vii) absence of molecular contamination indications. Together, these multiple lines of evidence strongly suggest that the analysed compounds derived from the fluid inclusions, while a minor contribution of organic compounds from the rock matrix cannot entirely be ruled out. This result adds to earlier studies, which demonstrated that fluid inclusions form closed systems that can preserve molecules even in billion-year-old metamorphic rocks.
Organic molecules detected by thermal decrepitation-gas chromatography–mass spectrometry and solid phase micro extraction-gas chromatography–mass spectrometry display major differences in diversity and abundance. Solid phase micro extraction probably provides a more authentic picture of the compounds contained in the fluid inclusions, because no heating to more than 50°C is applied before gas chromatography–mass spectrometry analysis. In contrast, thermal decrepitation resulted in abundant sulphur dioxide formation during heating to higher temperatures (250°C experiment), reflecting thermally driven artefact formation by reaction of the components in the interior of the fluid inclusions. Additionally, and even more important, the mild solid phase micro extraction offline approach can be applied on much greater sample amounts (gram vs. milligrams), resulting in detectable yields of trace compounds that are indiscernible with the thermal decrepitation approach.
The Dresser Formation formed in a hydrothermal environment. Hence, compounds entrapped in barite-hosted fluid inclusions may have been derived from abiotic sources. Indeed, gaseous compounds such as sulphur dioxide, carbon dioxide, hydrogen sulphide, carbonyl sulphide, carbon disulphide, and (methylsulphanyl)methane are known to be delivered to surface environments via volcanic outgassing. Functionalised lipid-like organic molecules such as ketones, aldehydes, carboxylic acids, and alcohols can be formed by Fischer–Tropsch-type processes under hydrothermal conditions. Further compounds of possibly abiotic origin are acetic acid and organic sulfur molecules (e.g. thiols, organic polysulphanes). These molecules may be synthesized in the presence of sulphide catalysts and with carbon disulphide or carbon dioxide as a carbon source. Extraterrestrial delivery by meteorites could have provided an additional source for many of the observed compounds (e.g. carbonyl sulphide, carbon disulphide, hydrogen sulphide, methanethiol, benzaldehyde, acetic acid, benzene, toluene, various aldehydes, and ketones).
While many compounds observed in the barite-hosted fluid inclusions from the Dresser Formation are consistent with an abiotic origin, the Dresser Formation also contains a variety of evidence for life. Thus, biology is another potential source for the observed compounds. In fact, organisms synthesise most lipids on modern Earth, and proportional carbon¹³ signatures of kerogen in the black barite (roughly –28 ‰) are in good accordance with biological carbon fixation. Furthermore, compounds such as hydrogen sulphide, carbonyl sulphide, carbon disulphide, (methylsulphanyl)methane, (methyldisulphanyl) methane, and thiols are typically formed during microbial sulphur cycling in modern environments, and there is isotopic evidence for the presence of sulfur-processing metabolisms during Dresser times.
Taken together, it is likely that the barite-hosted fluid inclusions contain mixtures of various abiotic and biotic compounds. Such contributions from different sources would plausibly explain the contrasting proportional carbon¹³ signatures of carbon dioxide in grey and black barites. Carbon dioxide released from grey barites exhibits a mean proportional carbon¹³ value of –6.3‰, which might be indicative of a magmatic source (typically between −2 and −8‰). In contrast, lower proportional carbon¹³ values of −10.3‰ in carbon dioxide from black barites might fingerprint a biomass-derived carbon component that had been converted to carbon dioxide via Bacterial and/or thermochemical sulphate reduction before it was absorbed and transported by fluids. The processing, re-distribution, and mixing of fluids from different sources is well known from modern and ancient hydrothermal systems (hydrothermal pump).
It is widely assumed that hydrothermal processes fuelled primeval life on Earth, but it is difficult to pinpoint the exact nature of such relationships in the Archaean rock record. The fluid inclusion-bearing black barites are interbedded with Stromatolites, suggesting that hydrothermal fluids may have influenced the ancient Microbial communities. Indeed, many compounds discovered in the barite-hosted fluid inclusions (e.g. carbonyl sulphide, carbon disulphide, acetic acid, (methylsulphanyl)methane, (methyldisulphanyl)methane, thiols, methane) would have provided ideal substrates for the sulfur-based and methanogenic microbes previously proposed as players in the Dresser environment. For instance, acetic acid may have fuelled acetoclastic methanogenesis, while organic sulphides such as methanethiol and (methylsulphanyl)methane might have served as substrates for fermenting methanogenic Bacteria. This hypothesis is in full agreement with isotopic evidence indicating the existence of methanogenic and sulphur-cycling Microbes in Dresser environments. The activity of sulphate reducing or sulphur disproportioning Bacteria could also account for the presence of abundant pyrite in the originally sulphidised Dresser Stromatolites. Thus, Mißbach et al.'s findings provide a strong clue that Microbial life associated with the black barites of the Dresser Formation was (partly at least) fuelled by hydrothermal fluid flow.
In addition to potential nutrients and/or substrates, hydrothermal fluids captured in the Dresser fluid inclusions contain molecules closely related to putative key agents in the emergence of life. It has been proposed that carbon monoxide and methanethiol can react in the presence of catalytic metallic sulphides to methyl thioacetate. This compound, also known as activated acetic acid, was proposed as being important for the formation of lipids under primordial conditions and as an energy source for early Microbial metabolisms. Whereas this highly energetic molecule is readily hydrolysed and cannot be preserved over geological time, our data evidence the presence of its stable building blocks, methanethiol and acetic acid, in the Dresser fluids. In other words, essential ingredients of methyl thioacetate, a proposed critical agent in the emergence of life, were available in the Dresser environments.
Mißbach et al.'s data provide the first detailed picture of the organic composition of primordial fluids that had evidently been available for the ancient Microbes roughly 3.5 billion years ago. These fluids delivered ample catabolic substrates for chemoheterotrophic metabolisms. In addition, they might have conveyed the building blocks for chemoautotrophic carbon fixation and, thus, anabolic uptake of carbon into biomass. Taken together, Mißbach et al.'s data strongly support the idea that hydrothermal fluids supplied a fertile substrate for early Microbial life on Earth.
See also...



Online courses in Palaeontology.
Follow Sciency Thoughts on Facebook.
Follow Sciency Thoughts on Twitter.