The Ediacaran–Cambrian boundary, approximately 541–539 million years ago, is widely recognized as a juncture of exceptional ecological and evolutionary importance. At around this point, the fossil record is permanently transformed by the appearance and radiation of diverse biomineralizing and agglutinating forms. This switching-on of the ‘shelly’ fossil record approximately corresponds with an increase in the degree and complexity of bioturbation, substantial shifts in the nature of biogenic sediments, a disappearance of macroscopic Ediacara-style preservation, and major changes in thecomposition of Acritarch assemblages. Identification of such ecological or evolutionary perturbations is heavily reliant on taphonomic continuity; in other words, the factors governing fossil preservation should not substantially change through the time interval of interest. If they do, then the traceability of lineages/taxa can be seriously compromised. The coincident opening and closure of several key taphonomic windows across the Ediacaran–Cambrian transition obscures the precise tracking of taxonomic ranges from this crucial interval. At present, only a handful of taxa known from body fossils are convincingly shown to span the boundary. The apparent disconnect in the body fossil record is contrasted by the relatively unbiased trace fossil record, which instead documents a signal of continuity between late Ediacaran and earliest Cambrian benthic Bilaterian behaviour. Before a precise description of the magnitude, timing and nature of this transition can reasonably be achieved, there is a pressing need for an improved accounting of non-biomineralising taxa in order to discriminate genuine macroevolutionary patterns from localised signals or taphonomic shortfalls. Small carbonaceous fossils offer one means of tracking the Ediacaran–Cambrian transition without the associated biases of mineralisation. Even under relatively indifferent taphonomic circumstances, cell walls, cuticle, and other recalcitrant components of non-biomineralising organisms can be recognisably preserved. The widespread preservation of small carbonaceous fossils has recently been demonstrated from regions and time-intervals where other, more ‘exceptional’ evidence of non-biomineralising taxa is lacking.
Cochleatina is especially interesting in that it is preserved in substantially different depositional environments to iconic boundary-spanning taxa such as Cloudina. Despite this, Cochleatina has so far been neglected from discussion of Ediacaran ‘survivors’, and so warrants renewed attention, particularly in the context of recent debate on rates of turnover, extinction and the nature of the Ediacaran–Cambrian transition.
Cochleatina is a coiled carbonaceous fossil formed as a spiral-shaped ribbon ornamented with fine serrations. Examples of this fossil were first figured among acid-extracted material from the Ediacaran of the Ukraine in the 1970s, but were initially interpreted as simple coiled filaments and ascribed to the filamentous formtaxon Volyniella (albeit as a new species). Three further species were later added based on material from the Rovno (latest Ediacaran or earliest Cambrian) and Lontova (Cambrian) formations in Belarus, Lithuania and Latvia, but remained assigned to Volyniella until Cochleatina was established as a new genus to circumscribe these morphologically distinct fossils in 1983. Several succeeding studies mentioned or figured Cochleatina from sediments in Baltica and Siberia, but with no substantial revision until a major redescription and analysis in 1995 in which the four currently accepted species were amended: Cochleatina canilovica, Cochleatina rara, Cochleatina rudaminica and Cochleatina ignalinica.

Examples of Cochleatina canilovica from the Ediacaran of the Volyn region of Ukraine. Scale bar represents 100 μm. Mikhail Burzin in Slater et al. (2020).
More recent reports of Cochleatina, recovered among Acritarch preparations, have expanded its known geographic range beyond Baltica and Siberia to Avalonia and Gondwana. Attempts to pin Cochleatina to the tree of life have been wide-ranging. Several authors have proposed a Metazoan affinity (among the Annelids or Molluscs), a premise which would clearly have significant implications if confirmed or refuted.
Slater et al. describe new material of Cochleatina from Ediacaran sediments of Estonia (Kotlin Formation) and Ukraine (Krushanovka Formation). They further discuss the broader significance of this small carbonaceous fossil taxon in light of its status as a credible Ediacaran–Cambrian ‘survivor’, in the context of recently revised stratigraphy and its emerging palaeobiogeographical distribution. Slater et al.We further examine and test previous hypotheses for the biological affinity of Cochleatina, and propose new models for its possible mode of life.

Palaeogeographic distribution of fossil occurrences of Cochleatina sp. (A) Localities in Baltica where Cochleatina sp. have been recovered; (1) outcrop, Finnmark, Norway; (2) Toila 77 and Meriküla F169 drillcores, Estonia; (3) Ludza drillcore, Latvia; (4) Vishki drillcore, Latvia; (5) Butkunay drillcore, Lithuania; (6) Svedasay drillcore, Lithuania; (7) Drukshyay drillcore, Lithuania; (8) Tvere cius drillcore, Lithuania; (9) Stradech-17 drillcore, Belarus; (10) various cores from Volyn, Ukraine (e.g. drillcore No. 1562, Il’pan); (11) various cores and outcrops from Podillya, Ukraine (drillcores: Bolotino, Vapnyarka No. 18, Malaya Sloboda No. 4, Bagovitsy No. 3, Pechora No. 2, Krushanovka No. 1, Zarechanka No. 11664; outcrops: Studenitsa village No. 202, Bakota village No. 238); (12) drillcore No. 700, Podillya, Ukraine; (B) distribution of palaeocontinents during the Ediacaran–Cambrian transition showing reported occurrences of Cochleatina sp., mainly from Baltica, but also Siberia, Avalonia and peri-Gondwanan terranes. Slater et al. (2020).
The Kotlin Formation is widely developed across the Baltic States on the East European Platform, and equivalent strata occur from Poland in the west, to the margin of the Baltic craton in the east. In Estonia, the Kotlin Formation is known exclusively from subsurface drillcore material, the nearest outcrop being on Kotlin Island (Russia) in the Gulf of Finland. The Kotlin Formation comprises a relatively homogeneous package of sediments composed predominantly of finely laminated grey, illite–smectite mixed-layer clays, with occasional interbeds of fine-grained sandstone and siltstone. Due to a relatively shallow burial depth and quiescent regional tectonic history, Kotlin strata have experienced negligible thermal alteration over their more than half a billion year history. In Estonia, the Kotlin Formation conformably overlies the coarser-grained sandy sediments of the Gdov Formation, and is in turn overlain by the correspondingly sandstone-rich Voronka Formation. Together, this package of Ediacaran sediments rests unconformably on a weathered crystalline basement.

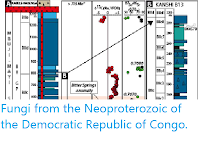
Ediacaran–Cambrian stratigraphy of Estonia and Ukraine (Podillya region). Red stars indicate position of samples analysed in Slater et al.'s study. Slater et al. (2020).
Despite its relative homogeneity, the Kotlin Formation in Estonia is partitioned into three subdivisions. The lowermost Jaama and uppermost Laagna members comprise relatively homogenous grey clays, whilst the middle Merik€ula Member can be distinguished by its visible fine-scale intercalations of sand, silt, and clay (‘varve-like’ appearance), abundance of sapropel films, and macroscopic ‘Vendotaenid’ fossils on bedding planes.
The Kotlin Formation was deposited in a shallow-marine pericratonic basin. Some authors have proposed brackish or even freshwater conditions within a basin with restricted circulation, based on suggestive boron concentrations in mudstones, localized absence of ‘Ediacara-type’ macrofossils, and a paucity of trace fossils. Certain regions where the Kotlin Formation developed, however, show clear evidence of marine deposition, and the extent of freshwater/brackish influence remains controversial.
The Kotlin Formation shares its name with the regional chronostratigraphic Kotlin stage, which in Estonia encompasses the Gdov, Kotlin and Voronka formations. Although once placed relatively deep within the Ediacaran System, the Kotlin Formation is now thought to have been deposited during the terminal 10 million years of Ediacaran time, based on correlation with strata from the Lublin Slope (Poland), Podillya (Ukraine), Urals and White Sea region (Russia) where uranium-lead zircon dates from volcanic tuff horizons have yielded lower boundary ages in the range of 551–548 million years old (zircon is a mineral formed by the crystallisation of cooling lavas, when
it forms it often contains trace amounts of uranium, which decays into (amongst other things) lead at a
known rate; since lead will not have been present in the original zircon, it is possible to calculate the age of a zircon crystal from the
ratio between these elements).
Ediacaran sediments of the Krushanovka Formation (Kanilovka Series) from Ukraine represent broadly coeval deposits, also belonging to the Kotlin regional stage Note that the Kanilovka Series of Podillya (alternatively Podolia) is not to be confused with the Kanilovka Formation of Volyn from which specimens of Cochleatina have been reported elsewhere in Ukraine. The Krushanovka Formation is widely known from drillcore in the Podillya region of Ukraine, and comprises a series of fine-grained, greenish-grey to white sandstones with substantial interbeds of reddish siltstones and claystones in its upper parts. The formation rests conformably on the Zharnovka Formation (a sequence of coarse to fine-grained sandstones) and is capped by the overlying Studenitsa Formation (predominantly coarse to fine-grained sandstones with occasional siltstones).
There are two recognized subdivisions of the Krushanovka Formation: a lower (roughly 45 m thick) Kryvchany Member, and an upper (about 15 m thick) Durnyakovka Member. The Kryvchany Member is generally coarser, with a larger proportion of sandstones, while the Durnyakovka Member is dominantly composed of distinctive red siltstones with occasional coarse sandstone beds. Deposition occurred in a shallow-marine basin with storm influence.
Sampling for microfossils targeted the most fine-grained lithologies (mudstones and siltstones) from both areas. In Estonia (Merik€ula Member of the Kotlin Formation), Slater
et al. processed a total of 31 samples: 11 from the Maidla 75A drillcore; 2 from the Maidla F-238 drillcore; 6 from the Toila 77 drillcore; and 12 from the Meriküla F-169 drillcore. From the Podillya region of Ukraine, a total of 5 samples were processed from the Durnyakovka Member of the Krushanovka Formation, drillcore No. 700. Estonian cores are housed at the
Tallinn University of Technology Institute of Geology core-storage at Särghaua (Estonia), and samples from drillcore No. 700 (Podillya, Ukraine) are hosted at the
Institute of Precambrian Geology and Geochronology of the
Russian Academy of Sciences in Saint Petersburg. Small carbonaceous fossil processing and examination followed a gentle, low-manipulation hydrofluoric acid maceration procedure aimed at the recovery of larger, delicate forms, otherwise destroyed by standard palynological processing.
Slater et al.'s processing recovered a total of 103 individual Cochleatina specimens, of which 70 are from the Estonian Kotlin Formation, and 33 come from the Ukrainian Krushanovka Formation. The majority of specimens were recovered from a small number of highly productive samples; Estonian specimens were recovered from a depth of 186–187 m in the Maidla 75A drillcore, 180 m depth in Maidla F-238 drillcore, 153 m in the Toila 77 drillcore, and 119.4 m from the Meriküla F-169 drillcore, whilst those from the drillcore No. 700 in Podillya, Ukraine were sourced from a productive layer at 184 m depth. Both the Estonian and Ukrainian samples of Cochleatina exhibit substantial taphomorphic variation. In the Estonian samples, all Cochleatina-bearing horizons produced masses of sapropel sheets, alongside occasional Vendotaenids and filamentous microbes. Productive samples from Ukraine were also associated with sapropel sheets, but at substantially lower levels.

Cochleatina from the Kotlin Formation, north-east Estonia. Specimens (A)–(F), (H)–(J), (L)–(O), (Q)–(S) from 153 m depth in Toila 77 drillcore; (G) from 180 m depth in Maidla F-238 drillcore; (K) and (P) from 187 m in Maidla 75A drillcore. Tallinn University of Technology acquisition numbers (GIT): (A) 831; (B) 842; (C) 837; (D) 838; (E) 836; (F) 843; (G) 850; (H) 841; (I) 828; (J) 842; (K) 851; (L) 841; (M) 829; (N) 833; (O) 838; P, 851; (Q) 841; (R) 839; (S) 832. Scale bar represents 100 μm. Slater et al. (2020).
Specimens from the new Estonian Kotlin assemblage are preserved as flattened spirals or incomplete sections of a spiral fused to sapropel films (sheets of relatively featureless organic matter, sometimes with identifiable filaments superimposed and variably fused together). These sapropel films are interpreted as compacted and variably fused sedimentary organic material and/or benthic mats. Specimens consist of a coiled ribbon; coils reach 540 μm in maximum width and display a continuum of morphologies, ranging from tightly wound bobbin-like configurations to more open spiral forms. The ribbon narrows towards the centre of the spiral and is a complex of four distinct longitudinal zones running the entire ribbon length. Thin, sharply pointed serrations project from the first inner zone, directed away from the centre of the coil, though these serrations are often obscured by the underlying organic sheet. Other zones are discernible by their thicknesses. Basal portions are either broken, or alternatively, where fused to a sheet, the ribbons have no obvious termination but instead fade into the sheet material.

Cochleatina from the Kotlin Formation, north-east Estonia. (D)–(L), specimens adhered to large sapropel sheets; (D), (F), (H), (K), and (L) are clustered Cochleatina, note that within each cluster coils are at approximately the same size, shape, and thickness. Specimens (A), (B), (D), (F), (K), (Q)–(S) from 189 m depth in Maidla 75A drillcore; (C), (E), (G), (H), (J), (L)–(P), (T) from 153 m depth in Toila 77 drillcore; (I) from 180 m depth in Maidla F-238 drillcore. Tallinn University of Technology acquisition numbers (GIT): (A) 845; (B) 846; (C) 840; (D) 848; (E) 832; (F) 853; (G) 838; (H) 835; (I) 850; (J) 852; (K) 849; (L) 854; (M) 829; (N) 842; (O) 834; (P) 830; (Q) 845; (R) 847; (S) 844; (T) 852. Scale bars represent: 100 μm; (A)–(F), (M)–(T); 200 μm (G)–(L). Slater et al. (2020).
The new Ukrainian Cochleatina as individual isolates (with one possible exception no clusters were recovered) and were never found in attachment to larger organic sheets. The coils reach 320 lm in maximum width. Like the Estonian specimens, the ribbons are divided into four discernible zones which narrow towards the centre of the spiral. The ribbons are optically darker than their counterparts from the Kotlin Formation, especially the first and third zones of the ribbon which are opaque in most specimens. Serrations emanating from the inner first zone of the ribbon are also prominently visible in the majority of specimens. The ribbon tip has a brush-like termination of fibrous projections between 5 and 15μm in length.

Cochleatina from the Krushanovka Formation, Podillya, Ukraine. Specimens sourced from a productive layer at 184 m depth within drillcore No. 700. Tallinn University of Technology acquisition numbers (GIT): (A)–(G), 855; (H)–(J), 856. Scale bar represents 100 μm. Slater et al. (2020).
The new specimens from Estonia and Ukraine are assigned to Cochleatina canilovica on the basis of their consistent spinose serration, ribbon oriented perpendicular to the bobbin axis, and four broad ribbon zones, features which are lacking in other taxa. Both the Estonian and Ukrainian assemblages are consistent with the currently known range of Cochleatina canilovica which is reported from the Kotlin regional stage of the late Ediacaran, and the lowermost part of the Rovno regional Ediacaran/Cambrian stage. Although Cochleatina has been reported from elsewhere in the Baltic region, these are the first reports from Estonian strata.

Schematic diagram of Cochleatina canilovica, including terminology of ribbon morphology used by Slater et al. The ‘first zone’ comprises the dark innermost part of the coil, and is fringed with marginal serrations that point away from the centre of the spiral. The ‘second zone’, where preserved, is a thin, filmy part of the ribbon which is typically overlain by the spines emanating from the first zone. The ‘third zone’ is of similar construction to the first zone (dark, sclerotised) but lacks any serrations and may be separated from the second zone by a ‘perforation zone’ toward the basal portion of the ribbon. The ‘fourth zone’ (frequently damaged or missing) is a thin, filmy region, similar to the second zone. Slater et al. (2020).
The new assemblages of Cochleatina from Estonia and Ukraine differ in a number of aspects. For example, serrations appear more pronounced in the Ukrainian specimens. This, however, appears to be purely taphonomic; serrations are present in all well-preserved Kotlin Cochleatina, but are simply less prominent due to the obscuring presence of the underlying/fused organic sheet. Cochleatina from the Krushanovka Formation exhibit darker ribbons (particularly in zones one and three), however, this can be explained by variations in local post-depositional burial histories (e.g. different degrees of thermal alteration). When these taphonomic considerations are taken into account, it is clear that both assemblages of Cochleatina exhibit the same underlying morphology.
Among the more complete specimens of Cochleatina recovered from the Kotlin Formation are a notable subset that occur as clusters, consisting of three coils adhered to the same carbonaceous sheet. The sheets are interpreted as the compacted remains of benthic organic material. No more than three coiled elements per cluster are seen, even on more extensive sheets. Within clusters, some coils are incomplete, and some partially overlap. Clusters can comprise tightly-wound bobbin-like and uncoiled forms, but within each cluster the coils are always of the same (potentially ontogenetic) stage/type. The asymmetry of the ribbon zones, in particular the overlap of the serrations, reveals that the coils occur as enantiomorphs (both right-handed and left-handed forms/chirality), which can co-occur in the same cluster. Occurrence as triplet clusters is an unexpected and novel insight into Cochleatina morphology. It is possible that the ‘individual’ Cochleatina reported in previous studies have been selectively disaggregated during more intensive, conventional palynological processing; indeed, low-manipulation processing appears to be essential for recovery of these delicate clusters. Since these Cochleatina are all at the same stage or type within a cluster, they are unlikely to represent fortuitous superposition via currents or fall-out from the water column. Either these clusters represent groups of three similar individuals from a population with a benthic ecology, or were clustered prior to sinking from suspension, or are the recalcitrant components of a single organism that has otherwise decayed away.
Previous suggestions for the biological nature of Cochleatina have been broad ranging, reflecting the dearth of suitable fossil or modern analogues (a problem shared with many Ediacaran fossils). Proposed affinities have included the coiled ‘elaters’ of Bryophyte-grade Plant spores, defensive ejectosomes of Cryptophyta and subcomponents of a macroscopic Alga. Homology with the elaters of Liverwort, Hornwort and Equisetum spores can be ruled out on both functional grounds (the ribbons of Cochleatina are solid with no internal cavity, and therefore unsuitable for extension and retraction via hygroscopic turgor), and the fact that spores assignable even to stem-Rmbryophytes are not otherwise known until the Ordovician. The coiled ribbon-like ejectosomes of Cryptophyta bear a superficial resemblance to Cochleatina but are intracellular organelles, orders of magnitude smaller than Cochleatina, making even an analogous function improbable. Similarly, the serrated filamentous ejectosomes of Helicosporidial cysts are somewhat similar in form to Cochleatina, but are less than ten microns in size.

Comparative extant and fossil analogues for Cochleatina. (A) Coiled elaters found in triplets on Elaterites triferens Plant spores (Pennsylvanian). (B) Scanning electron micrograph of dehisced Helicosporidial cyst (parasitic Green Algae) showing uncoiled filamentous cell bearing barbed serrations. (C) Reconstruction of the ribbon-like ejectosome of Cryptophyta Algae (intracellular scale). (D)–(E) Scanning electron micrographs of the Protozoan trapping structure of the Corkscrew Plant Genlisea repens (Angiosperm); (E) close-up of (D) showing serrated coils through which prey enters. (F) Redkinia spinosa from the Ediacaran of north-west Russia, inset shows enlargement of serrations. (G)–(H) Coiled organic sheets found in early Cambrian (Terreneuvian) cherts. (I) Paired coiled radula of the extant Mollusc Plawenia sphaera. (J) Coiled anterior region of the Ciliated Protist Stentor. Scale bars represent: 225 μm (A); 7.5 μm (B); 1 mm (D, F); 100 μm (E); 20 μm (G–H), 200 μm (I); 50 μm (J). Slater et al. (2020).
Cochleatina specimens have been reported in rare instances adhering to the macroscopic fossil ‘Alga’ Kanilovia insolita from the ‘Kotlin’ regional stage of Ukraine. This association with Kanilovia insolita (itself a problematicum) is intriguing, but whether the relationship is truly biological is difficult to ascertain; even if fortuitous superposition could be ruled out, there is the possibility that the Cochleatina were derived from epibionts or some other organism in association with Kanilovia insolita. Similarly, though the triplet associations of Cochleatina are probably biological, the attachment of Cochleatina to organic sheets (e.g. the Estonian material in this study) may or may not be biological. It is common among small carbonaceous fossil-style preservation for multiple overlapping organic constituents to become fused into a single layer during diagenesis. The sheets themselves preserve little discernible morphology, and although they could represent fragments of thalli (some have regular margins), they could alternatively be regarded as sheets of degraded and depolymerized organic matter (sapropel), to which the more recalcitrant Cochleatina are fused. The consistent within-cluster similarity of Cochleatina in these instances would at least suggest the coils themselves represent structures from a single individual, or individuals from a single population.
Elsewhere among the fossil record, some of the more densely coiled Cochleatina bear a superficial resemblance to sheet-like fossils preserved in Terreneuvian (lower Cambrian) hydrothermal cherts from South China, which can exhibit a tightly enrolled coil-like habit, the coils even occurring in ‘clusters’. These sheet-like fossils (interpreted as Animal cuticles) also bear a fine surface covering of hair-like or dentate projections. A more precise structural comparison to Cochleatina, however, is problematic; the surface spines on these silicified sheets are sparsely distributed hollow projections, quite unlike the regular rows of tooth-like serrations in Cochleatina. Moreover, Cochleatina is never found as distended, sinuous sheets or loops, but only occurs as regular coils. In instances where specimens are found on sheets there is no basal connection to a sheetmargin, indicating that Cochleatina cannot be the flattened enrolled margin of such a sheet or cuticle.
Although only a few of the previously proposed affinities for Cochleatina can be rejected outright, none offers a convincing basis for assigning it to any particular biological taxon. Nevertheless, there are other extant and fossil examples that serve to elucidate at least some of the characteristics that set Cochleatina apart. Notably, Cochleatina can be usefully compared to a variety of feeding structures seen in extant and fossil heterotrophs, from Protistan to Eumetazoan grade.
Comparisons have been made between Cochleatina and another serration-bearing carbonaceous fossil, Redkinia, which also occurs in Ediacaran deposits, both as microfossils and as bedding-plane visible mesofossils. It was initially proposed that Redkinia represented a disarticulated Polychaete jaw (i.e. a Scolecodont) and later, the mandible-like jaws of a stem-Arthropod; if the connection to Redkinia was established, it would potentially support a Bilaterian affiliation for Cochleatina. An earleir study highlighted the shared characteristics of Redkinia and Cochleatina, principally the first and second order serrations, which are somewhat similar to those seen in Cochleatina ignalinica, and considered the possibility of the latter evolving from the former based on their stratigraphic relationships (but questioned the ability of Cochleatina to have functioned as a feeding apparatus). It is also questionable whether the two structures (Cochleatina and Redkinia) are homologous; serrations are a deeply convergent morphological feature, and other than their carbonaceous habit, this is the only shared character which promotes any useful comparison.
A further likeness to Metazoan mouthparts is the broad similarity of Cochleatina to certain Molluscan radulae. In particular, the simple pairs of coiled radulae borne by certain Solenogastres are somewhat Cochleatina-like in overall appearance. Cambrian radulae are known from small carbonaceous fossils and from the radula-like mouthparts of Wiwaxia and Odontogriphus; Cochleatina substantially predates these occurrences. However, Cochleatina also lacks any belt-like arrangement of individual tooth-elements; the ribbon is a solid structure, with no joints or segments. Moreover, one of the species of Cochleatina (Cochleatina rudaminica) does not possess any serrations at all, making a radula-like function or homology unlikely.
Among extant organisms, a particularly useful comparison is with the giant (over 1 mm) single-celled Ciliate Stentor. Specifically, the coiled anterior region of oral cilia in Stentor is strikingly reminiscent of Cochleatina and reaches a similar size. These cilia are fused into flat, triangular plates and borne on a coiled basal membranellar band. Environmental shocks can lead to the membranellar band being sloughed off and detached from the main body of the Stentor. When shed, the membranellar band does not disaggregate, but remains fused as an isolated ribbon which contracts in the transverse direction to form an even more tightly wound coil. The microanatomy of Stentor (particularly Stentor coeruleus) has been studied in detail for its ability to regenerate, during which clusters of ciliary bands can form. Similar clustering can occur naturally during reproduction or during the sessile rest state, when numerous individual Stentor can attach adjacently to a substrate via their posterior holdfast. The main obstacle to analogy with Cochleatina is taphonomic. Without any obvious robust macromolecular extracellular components to the ciliary band, it is difficult to envisage how such a structure could produce the recalcitrant small carbonaceous fossil Cochleatina. It is possible that relatively labile structures could fuse to more resistant organic materials during diagenesis, forming a composite structure, and it is worth noting that seemingly decay-prone tissues are occasionally captured in Burgess Shale-type Lagerstätten (e.g. Ctenophores). Regardless of taphonomic issues, these similarities with Stentor demonstrate that complex small carbonaceous fossil structures like Cochleatina could, in principle, derive from Protists.
Another intriguing possibility is that the coils of Cochleatina functioned as a spiral Protozoan trap, analogous with the Protistan traps of extant Genlisea, the Corkscrew Plant. In Genlisea, specialized spiral rhizophylls with a narrow serrated slit serve to trap motile protists in the manner of an ‘Eel trap’. Progressively narrowed spirals or coils are prevalent among such traps in the broadest sense, including those of: ciliated predatory Protists (e.g. Stentor), helical Bryozoans, coiled Graptolites (e.g. Cyrtograptus and Monograptus turriculatus), the spiral traps constructed by Polychaetes, and even the bubble-traps of Whales. Viewed in this light, the multi-spiral and bobbin shaped forms of Cochleatina may represent multiple traps under continuous rejuvenation. Movement is key to predation; in a pre-muscular world (as also seen in Plant and Fungal predators), passive sit-and-wait trapping is expected to have been the standard feeding technique, with Protozoans as the primary target. Whereas Ediacaran Rangeomorphs may have extracted food via passive suspension, Cochleatina may represent a nextstep in luring self-propelled prey (perhaps aided by attractive chemotaxis as in Genlisea and carnivorous Fungi). Trapping of Protistan prey may be seen as part of a broader stepwise escalation of Uukaryovory and predation running from the Tonian to the Cambrian. Sponges (and Angiosperms and Fungi) also display rare instances of trap-based carnivory, but this style of hunting would have declined in importance in a world of increasingly motile Eumetazoan predators.
The oldest known Cochleatina are found in rocks of the Kotlin regional Baltic/Siberian stage. Under all schemes, the Kotlin is regarded as Ediacaran in age. The youngest Cochleatina are recovered from Fortunian strata of the regional Baltic Lontovan Stage, which probably corresponds to the latter half of Fortunian time based on its Acritarch and trace fossil contents (in particular the appearance of the Acritarchs Granomarginata prima and Asteridium tornatum along with trace fossils such as Treptichnus pedum, Gyrolithes and Monomorphichnus. The majority of reports, however, are sourced from the intervening ‘Rovno’ regional Baltic/Siberian stage. In the older literature, the Rovno was generally regarded as forming the uppermost division of the ‘Vendian’ System. It is currently unclear whether the Ediacaran–Cambrian boundary actually resides within the Rovno stage, however, in places the upper part of the Rovno Formation is clearly Fortunian (Treptichnus pedum and other typically basal Fortunian ichnofossils are found in the Rovno). While some recent schemes regard the entire Rovno stage as of earliest Fortunian origin, the generally accepted scheme places the lower parts of the Rovno in the Ediacaran and the upper portion, in which trace fossils of Cambrian aspect appear, in the Fortunian. Regardless of which scheme is used, Cochleatina ranges across the Ediacaran–Cambrian boundary.

Global stratigraphic range of body-fossils known to span the Ediacaran–Cambrian boundary compared to the range of Cochleatina sp. Temporal ranges for Cochleatina sp. from: (1) Estonia; (2) Podillya, Ukraine; (3) Volyn, Ukraine; (4) Belarus; (5) Lithuania; (6) Latvia; (7) Finnmark, Norway; (8) Burin Peninsula, Newfoundland; (9) Alborz Mountains, northern Iran; (10) Anabar Uplift, eastern Siberia. Note that ‘Redkino’, ‘Kotlin’, and ‘Rovno’ are informal regional stages of Ediacaran–Cambrian chronostratigraphy used in Baltica and Siberia. Slater et al. (2020).
The majority of Cochleatina specimens have been found in Ediacaran–Cambrian sediments of the Baltic Basin and Ukraine. Rare reports from beyond these sedimentary basins occur elsewhere on the palaeocontinent Baltica (Finnmark), as well as from the palaeocontinent Siberia, with isolated reports from Avalonia, and Iran. The current pattern is liable to change with increased exploration of undersampled regions, but taken at face value, the distribution of Cochleatina is centred on the margins of the Ægir Ocean, as well as adjacent peri-Gondwanan terranes.
Cochleatina demonstrates how small carbonaceous fossils can contribute to the emerging fossil record of Ediacaran–Cambrian ‘survivors’. Although all Cambrian taxa are necessarily derived from lineages that survived from the Ediacaran, the current picture of the Ediacaran–Cambrian boundary remains one of widespread fossil range truncation. Closer scrutiny, however, reveals a more complex pattern. ‘Terminal Ediacaran’ Cloudina, for example, is now known to range into the Cambrian, as do the ‘Ediacaran macrofossils’ Swartpuntia and Pteridinium, while the Cambrian Foraminiferan Platysolenites is documented in terminal Ediacaran strata. These are joined by a small but increasing number of Cambrian taxa which, on morphological grounds, appear to be examples of ‘Ediacara-biota’, but have thus far only been described from Cambrian rocks; e.g. Thaumaptilon and Stromatoveris. The current roster of ‘Ediacaran survivors’ is modest, but nonetheless significant. When combined with the continuity seen among the trace fossil record, an increasing case can be made for differential preservation, rather than purely extinction, accounting for at least some of the disconnect between Ediacaran and Cambrian biotas.
Cochleatina persisted for about 15–20 myr, from the latest Ediacaran to the latter part of the Cambrian Fortunian Stage. The range of Cochleatina encompasses possibly the most dramatic biotic transition in Earth history, spanning the close of the Proterozoic until their apparent disappearance in concert with the classical Cambrian ‘explosion’ of shelly Metazoans towards the end of the Fortunian. The Ediacaran was clearly a time of enormous experimentation in multicellularity, ecology and predation; an expansion of Bilaterians in the Cambrian may have marginalised previously successful modes of predation, perhaps accounting for the disappearance of forms such as Cochleatina. Shelly and trace fossil records probably represent a relatively reliable account of when various taxa and behaviours first appeared or disappeared during this part of the record; the same is not true for records from Lagerstätten, which are time-restricted and largely absent from this time-window. The challenge at the Ediacaran–Cambrian boundary is to distinguish fossil taxa that are taphonomically recalcitrant enough to preserve outside Lagerstätten conditions, and so stand a chance of exhibiting a global range in the first place. Small carbonaceous fossils appear to fulfil these criteria, at least during the latest Ediacaran and early Cambrian. Clearly the emerging distribution of Cochleatina reveals how small carbonaceous fossils can supplement a crucial geographical dimension to the problem of the Ediacaran–Cambrian biotic transition. Cochleatina is now known from four palaeocontinents and ten formations. Given this distribution, Cochleatina begins to enter the select realm of readily preserved, morphologically complex and widely distributed fossils from this time window, alongside iconic taxa such as Cloudina.
See also...












%20(1).png)