The Eukaryotic cell is strikingly distinct from its much simpler Prokaryote relatives, possessing not only a nucleus, but also a complex cytoskeleton, a sophisticated endomembrane system, and mitochondria, the last of these the result of an ancient endosymbiosis with a Proteobacterium. How this complex cell evolved has long been a puzzle, in part because of the lack of living intermediate taxa that would help determine the sequence in which these distinctive Eukaryotic characters appeared. The recent discovery of the Asgard Archaea, a group closely related to eukaryotes that possesses homologues of several Eukaryote genes, has heightened interest in this problem, as they represent, in a sense, the transitional forms that have long been sought. Nonetheless, there will always be evolutionary transitions that cannot be inferred using modern taxa, as is well illustrated by the Birds, whose evolutionary history would be a mystery without the rich fossil record of the Dinosaurs. The fossil record of early Eukaryotes traditionally has been dismissed as useless for reconstructing Eukaryogenesis, in part because the important transitions involve characters which are typically not preserved (e.g. the nucleus, mitochondria, cytoskeleton), and in part because the prevailing view of early Eukaryote evolution assumes these transitions took place long before the first Eukaryotes show up in the fossil record.
In a paper published in the journal Interface Focus on 30 March 2020, Susannah Porter of the Department of Earth Science at the University of California at Santa Barbara argues that there are fossil proxies that could be used for inferring the relative order in which some Eukaryotic characters evolved, and focus on four in particular: (i) excystment structures (medial splits or pylomes, i.e. circular openings in the cyst wall) which imply the capacity to form cysts; (ii) spines and pylomes, which require the cell to be able to change its shape and thus imply a complex cytoskeleton; (iii) sterane biomarkers which imply sterol synthesis; and (iv) evidence of Eukaryotes living in oxic habitats, which implies aerobic respiration, and thus the possession of mitochondria. Contrary to widespread assumptions, current evidence allows the possibility that some of these characters evolved significantly later than the first recognisable Eukaryotic fossils appeared, potentially enabling us to identify their time of appearance in the fossil record.
Before discussing the early Eukaryote fossil record, it is useful to review some common terms. Crown Group Eukaryotes are defined as the last common ancestor of all living Eukaryotes plus all of its descendants, both extinct and extant. This last common ancestor is commonly referred to as the Last Eukaryotic Common Ancestor. Stem Group Eukaryotes are those lineages that diverged prior to the appearance of the Last Eukaryotic Common Ancestor, but after the split with the group’s closest living relatives, a clade of Asgard Archaea in the case of Eukaryotes. Stem Groups are, by definition, extinct, and possess some, but not all, of the characters that define the Crown Group. Stem and crown groups together make up the Total Group. The term First Eukaryotic Common Ancestor, is often used to refer to the initial lineage of Total Group Eukaryotes, just after its split from its closest living relative. Eukaryogenesis refers to the interval between the First Eukaryotic Common Ancestor and the Last Eukaryotic Common Ancestor, when the characters that define the Crown Group evolved.

Diagram illustrating important terms and the evolution of selected characters that define modern Eukaryotes. Characters in italics are the focus of this paper. Note that the evolutionary sequence of characters that evolved after the First Eukaryotic Common Ancestor and before the Last Eukaryotic Common Ancestor cannot be resolved without the fossil record. Porter (2020).
The oldest widely accepted evidence of Eukaryotes is large (greater than 100 μm), spiny, ornamented, organic-walled microfossils found in latest Palaeoproterozoic rocks (about 1650 million years old). Throughout the Mesoproterozoic, organic-walled microfossil assemblages include a variety of Eukaryotic fossils, but it is not until the latest Mesoproterozoic and early Neoproterozoic that there is evidence for modern (i.e. crown group) Eukaryotes. This includes a handful of fossils plausibly assigned to various modern Eukaryotic supergroups, the first appearance of Eukaryotic steranes, and evidence of other Crown Group innovations like tests and biomineralised scales.
The most common explanation for these patterns is that while Crown Group Eukaryotes appeared more than 1.65 nillion years ago, they remained minor components of Mesoproterozoic ecosystems until their dramatic diversification in the early Neoproterozoic. Evidence offered in support of this view includes the absence of steranes from Mesoproterozoic rocks, which indicates that Eukaryotes must have been severely limited in abundance, and widespread ocean anoxia during this time, which means that early Eukaryotes, which require oxygen for aerobic respiration, must have been spatially restricted to the few habitats where sufficient oxygen was present. Porter evaluates the evidence for early crown group Eukaryotes and suggest instead that the data allow another end-member possibility: crown group Eukaryotes did not appear until the late Mesoproterozoic, and early Mesoproterozoic Eukaryotes had not yet acquired the capacity for aerobic respiration or sterol synthesis.
There are two end-member models of early Eukaryote evolution, which differ with respect to the age of the Last Eukaryotic Common Ancestor. If the Last Eukaryotic Common Ancestor appeared early (e.g. 1600–1800 million years ago), then Crown Group Eukaryotes must have inhabited Mesoproterozoic seas and are probably well represented among Mesoproterozoic Eukaryote fossil assemblages. Crown Group Eukaryotes would have been capable of aerobic respiration, and therefore would have lived in oxygenated environments (at least some of the time), and would have had the capacity to synthesize sterols. If, however, the Last Eukaryotic Common Ancestor appeared near the end of the Mesoproterozoic, then, depending on when in stem group evolution these characters evolved, it is possible that all early Mesoproterozoic Eukaryotes were anaerobic, unable produce sterols, or both. These different scenarios have different implications for our ability to trace Eukaryogenesis in the fossil record. If the Last Eukaryotic Common Ancestor appeared early, before the first recognisably Eukaryotic fossils, then those characters must have appeared in taxa that either did not leave a fossil record or are not recognisably Eukaryotic, making it difficult if not impossible to know the order in which they evolved. If, however, the Last Eukaryotic Common Ancestor appeared much later than the oldest recognisably Eukaryotic fossils, then it might be possible to track the appearance of different Crown Group characters through the Mesoproterozoic.

Models of early Eukaryote evolution. If the Last Eukaryotic Common Ancestor appears early, then Eukaryotes with cysts, a complex cytoskeleton, sterols and the capacity for aerobic respiration must have lived during Mesoproterozoic time. In this model, the lack of detectable steranes during this time would reflect limited abundance of Eukaryotes, particularly Crown Eukaryotes. Eukaryogenesis (orange box) would have preceded the record of recognisably Eukaryotic fossils (indicated by grey box). If the Last Eukaryotic Common Ancestor appeared late, then Mesoproterozoic rocks dominantly preserve Stem Group Eukaryotes, many of which may be anaerobic or lacked the ability to synthesise sterols, or both. Stages in Eukaryogenesis may be observable, given overlap with the fossil record. Porter (2020).
So, when did the Last Eukaryotic Common Ancestor appear? Unfortunately,
molecular clock estimates for the age of the Last Eukaryotic Common
Ancestor span a range of over 1 billion years, making it difficult to have much confidence in any particular estimate. Several arguments, however, favour a younger age for the Last Eukaryotic Common
Ancestor. First, older ages for the Last Eukaryotic Common
Ancestor seem to be driven primarily by the inclusion of Bangiomorpha pubescens, a presumed Bangiophyte Red Algal fossil. Many of these analyses used a now-outdated age for Bangiomorpha pubescens (1198 million years versus the recent report of 1.047 billion year), and this presumably accounts for some of the discrepancy. In addition, it is also possible that Bangiomorpha pubescens is neither a Bangiophyte nor even a Crown Group Red Alga. Other more deeply diverging Red Algal species exhibit similar filamentous forms, similar multicellular holdfasts and packets of spores that form in a similar way to the wedge-shaped cells of Bangiomorpha pubescens, suggesting these characters could have evolved multiple times early in Rhodophyte evolution. Bangiomorpha pubescens is therefore perhaps better interpreted as a part of Total Group Red Algae, as it could represent part of the Stem Group, rather than the Crown Group, of this clade. Notably, the analysis by Diana Chernikova, Sam Motamedi, Miklós Csürös, Eugene Koonin, and Igor Rogozin, assigned Bangiomorpha pubescens to Total Group Red Algae (using it to calibrate the split between Red and Green Algae), and yielded age estimates for the Last Eukaryotic Common Ancestor that were much less offset from those using Phanerozoic calibrations only (e.g. 1200–1400 million years with Bangiomorpha pubescens versus 1100–1300 million years without Bangiomorpha pubescens). This analysis used the now-outdated calibration age for Bangiomorpha pubescens (1100–1200 million years), suggesting that both a corrected age and more conservative taxonomic assignment for Bangiomorpha pubescens might remove the discrepancy altogether, with Phanerozoic and Proterozoic calibrations aligning in support of a younger (1100–1300 million years ago) age for the Last Eukaryotic Common Ancestor.

Selected molecular clock constraints on the initial divergence within crown group eukaryotes (i.e. the age of the Last Eukaryotic Common Ancestor). Estimates that do not use the fossil Bangiomorpha pubescens as a calibration point are 200–300 million years younger than those that do. Porter (2020).
Second, though estimates for the Last Eukaryotic Common Ancestor vary widely, there is consistent agreement among molecular clock analyses that the Eukaryotic supergroups diverged within 300 million years of the Last Eukaryotic Common Ancestor. Thus, if the Last Eukaryotic Common Ancestor appeared early (1600–1800 million years ago), then there should be evidence for Eukaryotic supergroups roughly 1300–1500 million years ago. Though the Mesoproterozoic is not as well sampled as the Neoproterozoic, thus far the picture seems to be that Eukaryotic supergroups diverged in the latest Mesoproterozoic and early Neoproterozoic, with fossils plausibly assigned to the Archaeplastida, Amoebozoa and Opisthokonta, appearing 1050–750 million years ago, along with other innovations widespread among living Eukaryotes (such as biomineralized scale microfossils; 811 million years ago). Thus, if Eukaryotic supergroups diverged 1050–750 million years ago, then these molecular clock analyses are telling us that the Last Eukaryotic Common Ancestor appeared around 1350–1050 million years ago.
Finally, models of phylogenetic diversification suggest it is highly unlikely that a Crown Group would survive at low diversity for approximately half its life (e.g. 800 million years) before it radiated. Rather, clades that survive to the present day (by definition any crown group) tend to start off with higher net rates of diversification relative to other (nonsurviving) clades. This is because clades that happened to have diversified rapidly early in their history are simply more likely to survive to become Crown Groups. It is not easy to persist at low diversity for hundreds of millions of years; there is just too much risk of going extinct. These phylogenetic models also indicate that after the Crown Group emerges, the Stem Group rapidly declines. This again reflects survivorship bias; if they did not decline rapidly, it is unlikely they would have gone extinct, which by definition, they must have, given that they are Stem Groups. Thus, the interval of overlap between Stem and Crown Group Eukaryotes should be short; if the Last Eukaryotic Common Ancestor did appear around 1.6–1.8 billion years ago, there should be few Stem Group Eukaryotes present by late Mesoproterozoic time. These results do not hold in special cases, e.g. when the Crown diversification follows a mass extinction, and, therefore, it is not easy to know how to apply them to the particular case of Eukaryotes. However, there is no obvious evidence for mass extinction in the Mesoproterozoic or early Tonian. Regardless, the null model suggests that once the Last Eukaryotic Common Ancestor had appeared, Crown Eukaryotes should have diversified rapidly. Thus, if Crown Group Eukaryotes diversified in the early Neoproterozoic, then the null model favours a younger (i.e. late Mesoproterozoic) age for the Last Eukaryotic Common Ancestor. Given reasons to favour a younger Last Eukaryotic Common Ancestor, Porter now turns to the fossil record and review the evidence for when important Eukaryogenic characters evolved.
Many Eukaryotes enter a resting stage during times of environmental stress, forming resistant-walled structures known as cysts. Encystation is so widespread, found among all the Eukaryotic supergroups, that it seems likely that the Last Eukaryotic Common Ancestor was also able to encyst, or least possessed a capacity to readily evolve cysts such that cysts evolved again and again within Crown Group Eukaryotes. Cysts are most easily recognised in the fossil record by the presence of excystment structures, pre-programmed openings by which the cells escape. Two types are common in Proterozoic fossils: medial splits, in which the cyst opens along an equatorial line often dividing the cyst into two separate hemispheres; and well-defined (typically circular) openings, known as pylomes. Fossils exhibiting medial splits are common in assemblages throughout the Proterozoic, including those with the oldest recognized Eukaryotes. Possible pylomes are reported in Dictyosphaera macroreticulata from 1744 to 1411 million years ago Ruyang Group, North China, with definite occurrence by 1100 million years ago (Leiosphaeridia kulgunica in the Atar/El Mreïti Group, Mauritania).

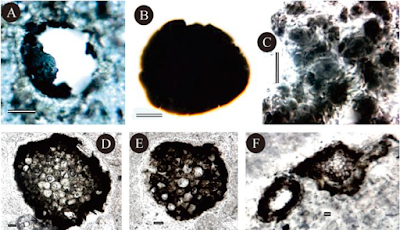
Examples of fossils and characters. (a), (b) Bangiomorpha pubescens from the late Mesoproterozoic Hunting Formation, Somerset Island, arctic Canada. Note cross-section in (b) showing distinctive wedge-shaped cells. (c), (f ) Excystment structures in Eukaryotic microfossils. (c) Circular pylome with partially detached lid, in Kaibabia gemmulella. (f) Medial split, in Leiosphaeridia crassa. Both (c) and (f) are from the late Tonian Chuar Group, Grand Canyon, USA. (d), (e) Indirect evidence for a complex cystoskeleton in Eukaryotic microfossils. (d) Irregularly distributed hollow processes in Tappania plana. (e) Spiny ornamentation in Dictyosphaera macroreticulata. Both (d) and (e) are from the early Mesoproterozoic Ruyang Group, North China. Porter (2020).
In Eukaryotes, the cytoskeleton is involved in controlling cell shape, cell movement, phagocytosis and intracellular trafficking. Though traditionally considered a diagnostic character of Eukaryotes, the discovery of actin and tubulin homologues in Bacteria and Archaea indicated cytoskeletal building blocks were already present in the First Eukaryotic Common Ancestor, and, in fact, recent discoveries of numerous homologues of cytoskeletal genes in Asgard Archaea suggest that the First Eukaryotic Common Ancestor had a dynamic actin cytoskeleton, perhaps even capable of phagocytosis. Nonetheless, many Eukaryotic cytoskeletal genes do not have homologues in the Archaea, suggesting that Eukaryotes have a much more complex cytoskeleton than their closest Archaeal relatives. It is difficult to know how to translate these genotypic differences into phenotypic differences we might observe in the fossil record, however. In the past, the presence of irregularly distributed, branching processes in Eukaryotic fossils has been used as evidence for a Eukaryotic cytoskeleton (in Tappania plana), but the presence of similar, irregularly distributed, long, sometimes branching protrusions extending from Asgard Archaeal cells recently described from culture suggests this might not be a uniquely Eukaryotic feature. Similarly, it is difficult to know whether complex ornamentation or the presence of spines in a fossil implies a complex (Eukaryotic) cytoskeleton or whether the First Eukaryotic Common Ancestor could have produced these with its more primitive cytoskeleton.

Proxy records for four characters of crown group Eukaryotes (coloured circles), and inferred age constraints on their appearances (coloured arrows): (a) cysts, represented by microfossils with excystment structures (medial splits or pylomes); and (b) the presence of a complex cytoskeleton, represented by microfossils with spines or pylomes, which indicate an ability of the cell to change its shape; (c) sterol synthesis, represented by sterane biomarkers; (d ) aerobic respiration, represented by the occurrence of Eukaryotes in oxic habitats (grey circles indicate evidence is ambiguous). Note that, with the exception of steranes, the absence of a proxy record is not taken to imply the absence of the character, age constraints in (a), (b), (d), show hard minimums but no maximums. Note also that age constraints in (a)–(d) do not take into account data from other records, e.g. fossil evidence for crown group Eukaryotes at 1.05 billion years. Porter (2020).
Thus, rather than focusing on proxies for a complex cytoskeleton, it might make sense to focus on features that the Eukaryotic cytoskeleton confers, recognising that some of these might have been present in the First Eukaryotic Common Ancestor. In particular, the ability for a cell to change shape, a key feature of the Eukaryotic cytoskeleton that is linked to the ability to phagocytose, might be inferred through several fossil proxies. Two have already been mentioned: evidence for an actively growing (and remodelling) cell wall (as in Tappania plana) and the presence of spines in cell walls or cysts, which probably formed via the extension of long, narrow protrusions of the cell membrane. Another proxy might be the presence of pylomes, as it might imply that the cell changed shape, squeezing through the hole to escape the cyst.
The distribution of sterols across the tree of Eukaryotes suggests that they were present in the Last Eukaryotic Common Ancestor, although it is possible that lateral gene transfer is at least in part responsible for this distribution. Archaea do not appear to have sterol genes, suggesting that the capacity for sterol synthesis appeared during the First Eukaryotic Common Ancestor–Last Eukaryotic Common Ancestor transition. (Note that while some bacteria produce sterols, their sterol synthesis genes are generally thought to have been acquired from Eukaryotes via lateral gene transfer). Thus far, biomarker studies of Mesoproterozoic rocks have failed to turn up convincing evidence of indigenous Eukaryotic steranes; this is the case even in units with diverse Eukaryotic fossils, in facies that would be favourable to sterol-producing Eukaryotes (i.e. oxic, nutrient-rich). Though this has generally been interpreted to indicate either an extremely low abundance of Eukaryotes or preservational bias, it may instead be that the absence of Eukaryotic steranes reflects the fact that sterol synthesis had not yet evolved. This would be consistent with a late appearance of Last Eukaryotic Common Ancestor (i.e. less than 1100 million years ago), but would also be consistent with an earlier appearance if the genes for sterol biosynthesis were transferred via lateral gene transfer among early Crown Group Eukaryotes. (Note that a recent molecular clock analysis concluded that some sterol biosynthesis genes were present in early Eukaryotes about 2.3 billion years ago (1.75–3.05 billion years ago). However, this does not imply that they were forming Eukaryotic sterols but rather might have been forming ‘protosterols’).
Today, mitochondria are essential for Eukaryotic respiration in aerobic habitats. Thus, it is reasonable to assume that evidence for aerobic respiration in early eukaryotes implies the presence of mitochondria in these organisms. (Note, however, that the converse is not true: evidence for an anaerobic lifestyle does not imply that these Eukaryotes lacked mitochondria.) Despite frequent acknowledgement in the literature that late Palaeoproterozoic and Mesoproterozoic Eukaryotic fossils are probably best interpreted as Stem Group Eukaryotes, there is a widespread assumption that nonetheless they must have been aerobic (and therefore possessed mitochondria). In part, this might reflect assumptions about the age of the Last Eukaryotic Common Ancestor, but it might also be based on the idea that aerobic respiration is required for the relatively large size and complexity of late Palaeoproterozoic and Mesoproterozoic Eukaryotic microfossils. Discoveries of anaerobic Eukaryotes over the last decade, however, have shown that this is not the case. Some anaerobic Protists (including free-living forms) can reach hundreds of micrometres in size and possess complex structures, including elaborate attachment sites, complicated cytoskeletons, large nuclei and thousands of flagella. In some cases, there is evidence that large size and complexity evolved within ancestrally anaerobic lineages, indicating that aerobic respiration is neither necessary to sustain large size and complexity, nor required to evolve it. Thus, the fairly large, ornamented Eukaryotic cells found in late Palaeoproterozoic and Mesoproterozoic rocks cannot be assumed to have been aerobic. (Whether their large size and complexity implies the presence of mitochondria per se, however, is more difficult to know).
A better proxy for aerobic respiration in early Eukaryote fossils is evidence that those Eukaryotes lived in oxygenated environments. Currently, the most widely used tool for reconstructing local redox conditions in an ancient water column is iron speciation, which uses the ratio of highly reactive iron versus total iron in a sample as a proxy for water column anoxia. Ratios of 0.22 or less are taken to indicate fully oxygenated water column, whereas highly reactive iron versus total iron ratios of 0.38 or higher suggest water column anoxia (ratios in between are ambiguous). Unless there is reason to think the organisms were infaunal, the presence of Eukaryotic microfossils in oxic samples should generally indicate that those organisms were aerobic. (While Eukaryotic fossils recovered from anoxic samples have traditionally been interpreted to be planktonic Eukaryotes that lived in the oxic surface layer of a stratified water column, it is also possible they were anaerobic and lived lower in the water column or on the seafloor). The link is not clear-cut, however, because both the iron speciation proxy and microfossil assemblages are integrated records, providing information about redox conditions and communities over some interval of time: samples that indicate an oxic water column might have witnessed short episodes of anoxia, and therefore might host fossils of anaerobic Eukaryotes. Conversely, the absence of Eukaryotes from oxic samples does not imply aerobic Eukaryotes did not exist, as they may be missing for other reasons, such as a lack of food, preservational bias or dilution due to high Cyanobacterial fossil abundance. Thus, no single sample should be taken as definitive evidence for (or against) aerobic Eukaryotes. But a consistent pattern across many samples and many units through time could provide compelling evidence for the absence of aerobic respiration.
Unfortunately, there are only a handful of studies in which iron speciation and fossil occurrence data come from the same samples, and the data are too sparse to come to any definitive conclusions about when aerobic Eukaryotes evolved. Nonetheless, unpublished work on the roughly 780–730 million year old Chuar Group (USA) suggests that the proxy may be useful: numerous oxic shale samples from throughout the Galeros Formation contain well-preserved Eukaryotic fossil assemblages that show the same species richness as those from anoxic samples providing good evidence for aerobic Eukaryotes at this time (not surprisingly) and suggesting there is no inherent preservational bias against organisms that lived in fully oxygenated water columns. Further back in time, the data become more difficult to interpret, and the evidence for aerobic Eukaryotes becomes less certain. In a palaeoecological study of the roughly 1100 Ma Atar/El Mreïti Formation of northwest Africa, there are only two fossiliferous oxic samples, only one of which preserves definitive Eukaryote species as minor components (less than 3% of the assemblage). However, the anoxic samples, which are much more numerous, are not dissimilar: although these can record abundances of Eukaryotes up to greater than 25% of the assemblage, the median Eukaryote abundance is nonetheless 0%. An analysis of drill core from the 1400 million year old Kaltasy Formation (Russia) revealed a suite of oxic samples, but definitive eukaryotes are rare or absent. This stands in contrast with more diverse assemblages from unknown redox habitats preserved in roughly coeval units from Australia and the USA. In the roughly 1450 million year old Roper Group, diverse Eukaryotes occur in environments interpreted to have been oxygenated, but iron speciation data are based on an outdated extraction method, and more recent research suggests that marine anoxia characterised the depositional basin of the Roper Group.
We know that First Eukaryotic Common Ancestor was an anaerobic organism, and that the oldest fossilised Eukaryotes lived in oceans that were dominantly anoxic. Thus, the default assumption should be that the earliest Eukaryotes to show up in the fossil record were anaerobic, with the onus on palaeontologists to show this is not true. The very limited data for Mesoproterozoic Eukaryotes discussed by Porter do not provide strong evidence either way: though a few Eukaryotic specimens are reported from oxic samples, the possibility that they reflect brief appearances of anaerobic organisms in anoxic conditions cannot be ruled out. A critical reading of the available data, therefore, would suggest that while aerobic Eukaryotes were present by roughly 800 million years ago, there is no definitive evidence that Eukaryotes occupied oxic habitats during the Mesoproterozoic. Contrary to widespread assumptions about early Eukaryotes, aerobic respiration, and therefore possibly mitochondria, might have been acquired late in Mesoproterozoic time, and late in stem group evolution.
The four characters can be mapped onto a phylogeny of early Eukaryotes, where the appearance of the Last Eukaryotic Common Ancestor is placed at 1100 million years. The limits of this approach are well illustrated: while the fossil record can provide definitive minimum age constraints on the appearance of these characters, cysts and a complex cytoskeleton by about 1650 million years ago, aerobic respiration (and thus mitochondria) by about 800 million years ago and sterol synthesis by 820 million years ago, it is more difficult to place maximum age constraints on the appearance of these characters. This relies on evidence of absence, which is particularly a problem with the fossil record, where preservational controls are always a concern. However, hypotheses regarding the timing of the origin of these characters remain testable using the rock record. For example, if continued sampling of well-preserved Mesoproterozoic and Palaeoproterozoic rocks consistently yields biomarkers, but never steranes, it may suggest that the strata in question predate sterol biosynthesis. Similarly, the timing of the evolution of aerobic respiration may be constrained by strata that consistently yield well preserved and abundant Eukaryotic body fossils in anoxic habitats but negligible occurrences in oxic habitats. While the absence of evidence in these scenarios does not necessarily provide definitive evidence that Eukaryotes lacked these distinctive characters, the simplest explanation is that these characters had not yet evolved.
The prevailing view of the early eukaryote fossil record is that the Last Eukaryotic Common Ancestor appeared by 1.6–1.8 billion years ago, and that the first Eukaryotes we see had the capacity for sterol synthesis and aerobic respiration. If this is true, then, given what we know about the Archean and Palaeoproterozoic fossil record, we have little hope of reconstructing Eukaryogenesis. But there is good reason to think this is not true. Molecular clock analyses allow the possibility that Last Eukaryotic Common Ancestor arose much later, a scenario favoured by current views that crown group diversification occurred in the latest Mesoproterozoic and Tonian. The consistent lack of detectable steranes in Mesoproterozoic rocks, especially in units that should host them, points to the possibility that Eukaryotic sterol synthesis had not yet evolved. Finally, there is no clear-cut evidence that Eukaryotes lived in oxic habitats during the Mesoproterozoic, permitting the possibility that aerobic respiration, and perhaps mitochondria, which are essential for aerobic respiration, was acquired late in Eukaryote evolution. Continued studies of body fossil and biomarker assemblages placed within a palaeoenvironmental and redox context may allow us to track Eukaryogenesis in the fossil record, and identify the environmental conditions that allowed the appearance of such complex life.
See also...



Online courses in Palaeontology.
Follow Sciency Thoughts on Facebook.
Follow Sciency Thoughts on Twitter.





















%20(1).png)